-
Call Now
1800-102-2727
Rearrangement reaction: Rearrangement Reaction, Rearrangement of Carbocations, Curtius Reaction, Claisen Rearrangement, Beckmann Rearrangement, Hoffmann Rearrangement, Pericyclic Rearrangement, Photochemical Rearrangement, Practice Problems and FAQs
How do you arrange your books and notebooks on your shelf?
I guess we all love being organized about our books, so that we can get easy access to any books whenever there is a requirement.
Imagine you have a well maintained and organized bookshelf, where physics, chemistry, math and english books have a separate columns.

Recently on your birthday, you received eight more chemistry books as a gift. But there is not enough space to accommodate all of them in the chemistry column.
What will you do in this scenario? Given you can’t create a new column.
Well, there are plenty of permutations and combinations through which you can rearrange them. Once you figure out what your requirements are, it will be easier to rearrange them in a proper order.
Similarly in organic chemistry, whenever a compound is exposed to external factors or to another compound or metal, the respective compound may become unstable. Hence, to undo this instability, sometimes they rearrange their molecules. This mechanism is called rearrangement reaction in organic chemistry. Let’s understand different types of rearrangement reactions and their mechanisms.
Table of content
- Rearrangement reaction
- Rearrangements of carbocations
- Curtius reaction
- Claisen Rearrangement
- Beckmann Rearrangement
- Hofmann Rearrangement
- Pericyclic Rearrangement
- Photochemical rearrangements
- Practice problems
- Frequently asked questions
Rearrangement reaction
Two distinct categories of organic chemical processes are referred to as "rearrangements." In a relatively short-lived intermediate, a rearrangement may involve the one-step migration of a H atom or a bigger molecular fragment.
The migration of a H atom or a bigger molecule fragment, however, may be one of the phases in a multi-step reaction known as a rearrangement.
The migratory group frequently attaches to an atom that was one of the direct neighbours of the atom to which it was initially linked in rearrangements. These types of rearrangements are known as [1,2] – rearrangements or [1,2] – shifts. The numerals 1 and 2 designate the subclass to which these rearrangements belong, and they can be thought of as sigma-tropic processes.
Rearrangements of carbocations
- Carbocations undergo several structural reorganisation shifts within the molecule..This movement of carbocation to attain stability from an unstable state is known as rearrangement of carbocations.
- whenever an alkyl halide, alcohol, or alkene is converted into a carbocation, the resultant carbocation may undergo rearrangement. The resulting carbocation will undergo additional chemical reactions to produce a final product with a different alkyl skeleton than the initial component. Carbocation rearrangements can be of two different types: hydride shifts and alkyl shifts.
- A carbocation may rearrange whenever it is created in a reaction. Only carbocations that are able to generate more stable forms will rearrange.
Shifting of 1,2 hydrogen
A chemical reaction known as the 1,2 Hydride Shift occurs when hydrogen travels from one carbon atom to another carbon in a molecule. Moving over greater distances is also feasible in a 1,2 hydride shift, which involves two neighbouring atoms.

Shifting of alkyl group (1,2 shift)
When a carbocation lacks a hydrogen atom that is present on the nearby carbon atom that is easily available for rearrangement, an alkyl shift happens. In certain circumstances, a hybrid shift does not result in a steady carbocation.

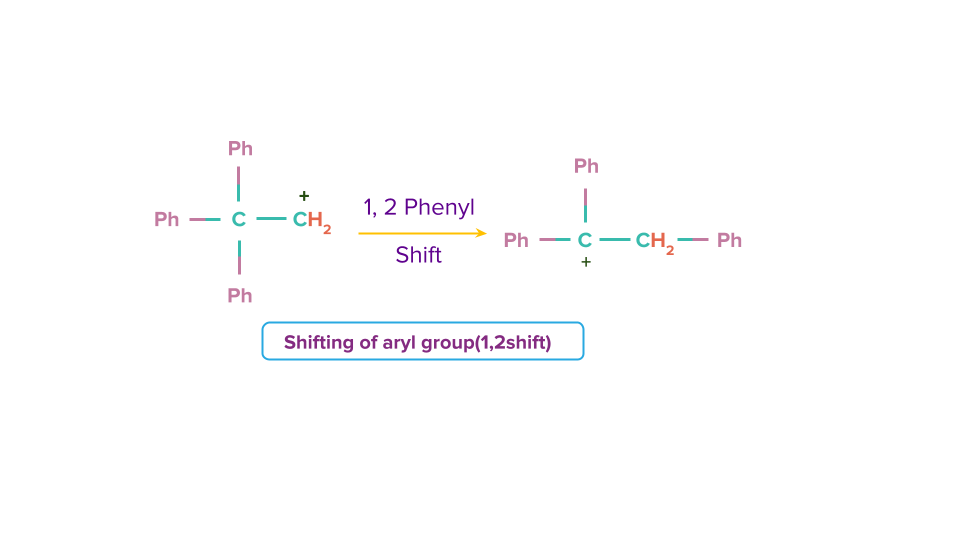
Shifting of aryl group
An aryl group in a carbocation moves from a neighbouring carbon atom to the carbon atom with the formal charge of +1 (carbon 2) in a carbocation rearrangement known as a 1,2-aryl shift (carbon 1)
Ring expansion
The moving component doesn't always have to be a methyl group! One intriguing instance is the formation of a carbocation next to a stretched ring, like a cyclobutane. Even if the CH3might move in this situation, shifting one of the ring's alkyl groups would be more advantageous and result in ring expansion and the production of a less strained, five-membered ring.

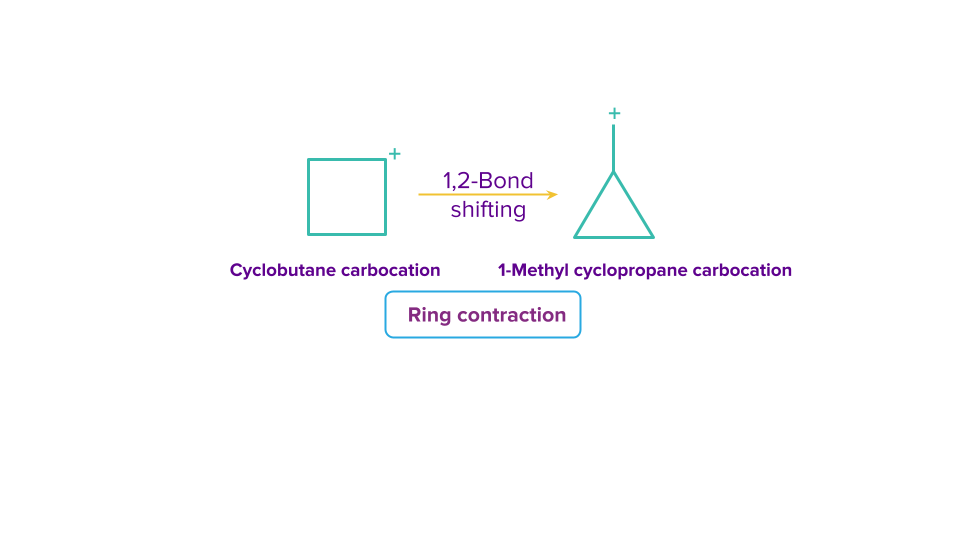
Ring contraction
Ring contractions are beneficial for converting bigger rings into smaller, more strained rings. While contractions are only distinguished by the reactive intermediate that accomplishes the contraction, expansions are categorised by the mechanism of expansion and the atom(s) added
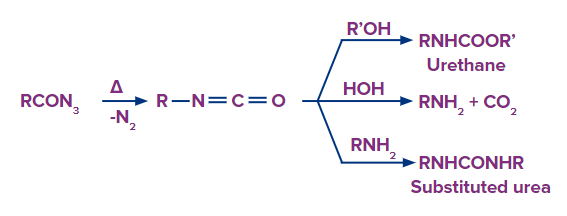
Curtius reaction
An acyl azide is heated in Curtius' reaction, losing nitrogen before rearranging to become an isocyanate.
The isocyanate undergoes further reactions to produce urethane, amine, or substituted urea when the process is conducted in an alcoholic or aqueous solution.
Curtius rearrangement is used to transform acyl azides into isocyanates, whereas Curtius reaction uses Curtius rearrangement to transform acids into amines, urethane, and substituted urea.
The following is how to obtain the acyl azide needed for the process.
Claisen Rearrangement
The first and slowest stage in the isomerization of allyl aryl ethers into ortho alkylated phenols is the conventional claisen rearrangement. The actual rearrangement step, a [3,3]-sigmatropic rearrangement, results in the formation of a cyclohexadienone. At the same time, three valence electron pairs shift.

Unable to be separated, the non-aromatic molecule cyclohexadienone rapidly tautomerizes to the aromatic and subsequently more stable phenol.
Allyl aryl ethers and allyl vinyl ethers undergo a thermal transformation in the claisen reaction. The closely related cope rearrangement may be viewed as its oxa-version. This reaction was first uncovered by Claisen on allyl vinyl ethers, and he later expanded his research to include the rearrangement of allyl aryl ethers to produce o-allylphenols.
Beckmann Rearrangement
An oxime is changed into an amide via the Beckmann rearrangement. Treatment of aldehyde or ketone with hydroxylamine makes it simple to produce an oxime. Ketoximes' OH group has the potential to leave the compound. Lactams are produced when cyclic oximes undergo the Beckmann rearrangement.
The combination of oxime production and Beckmann rearrangement successfully inserts an NH group between the carbonyl carbon and the alpha carbon, as shown by a comparison of the starting ketone's structure with that of the final product.
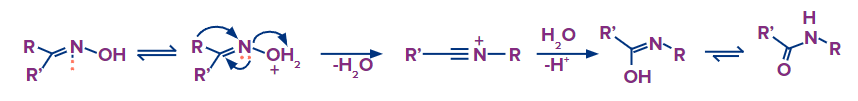
Because the end product, caprolactam, is the direct precursor of nylon 6, a versatile polymer with numerous applications, such as the production of fibres for carpets and other textiles, Beckmann rearrangement of the oxime of cyclohexanone is carried out on a very large scale industrially. Both the acid catalyst and the reaction's solvent are concentrated sulfuric acid.
Hofmann Rearrangement
A primary amide undergoes the Hofmann rearrangement when it is treated in water with bromine and the hydroxide ion, resulting in the formation of an amine in which the carbonyl group of the original amide has been removed.
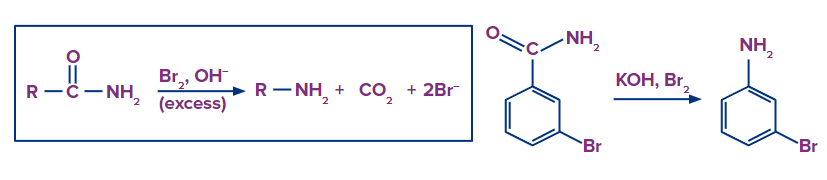
As a result, the carbon chain is cut by one atom as a result of the Hofmann rearrangement, and the functional group is changed from an amide to an amine. A comparable mechanism to the Beckmann rearrangement is used for the Hofmann rearrangement.
Pericyclic Rearrangement
Pericyclic reactions are those that result from a coordinated cyclic electron shift. Two essential characteristics of a pericyclic reaction are stated in this definition.
The first point is that there is a concerted reaction. Without intermediates, reactant bonds are broken and product bonds are produced in a concerted reaction.
A cyclic shift of electrons is the second crucial aspect of pericyclic processes. Pericyclic literally means "around the circle." The cyclic shift of electrons is where pericyclic words originate. Thus, a cyclic transition state involving the pi bonds characterises pericyclic processes.
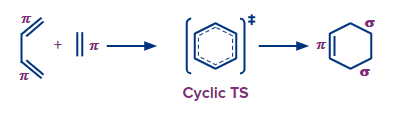
Heat or UV light provide the energy needed to activate pericyclic processes. The two induction techniques frequently result in products with opposite stereochemistry because pericyclic reactions are stereospecific.
Any pericyclic reaction has three distinct characteristics that are closely connected. which are
Pericyclic reactions are triggered by thermal energy or UV radiation, respectively. However, many reactions that call for heat are not started by light, and the opposite is also true.
how many pi bonds are engaged in the reaction.
The reaction's stereochemistry
Photochemical rearrangements
It is known that several photoreactions can interconvert isomeric substances. Although "rearrangement" is a more generic term than "isomerization," this distinction is unimportant for the reactions covered by photochemical rearrangement.
We will group primary photochemical rearrangements into the following categories for ease of reference.
- Cis trans isomerization
- Sigmatropic rearrangements
- Electrocyclic rearrangements
- Structural rearrangements which result from intramolecular cycloadditions.
All four of these classes can be thought of as special examples of pericyclic rearrangements in a broad sense, and for concerted reactions, they can all be handled using a single framework that is governed by guidelines derived from orbital symmetry concerns.
Practice problems
Q.1 Which of the following is not an example of a rearrangement reaction?
- Curtius reaction
- Clemension reaction
- Aldol condensation reaction
- Alkyl shift
Answer: (C)
Solution: Two distinct categories of organic chemical processes are referred to as "rearrangements." In a relatively short-lived intermediate, a rearrangement may involve the one-step migration of a H atom or a bigger molecular fragment. The migration of a H atom or a bigger molecule fragment, however, may be one of the phases in a multi-step reaction known as a rearrangement.
An organic process known as aldol condensation occurs when an enolate ion combines with a carbonyl compound to produce either a hydroxy ketone or a hydroxy aldehyde, which is then dehydrated to produce a conjugated enone. In order to create carbon-carbon bonds, aldol condensation is a crucial step in the synthesis of organic compounds.
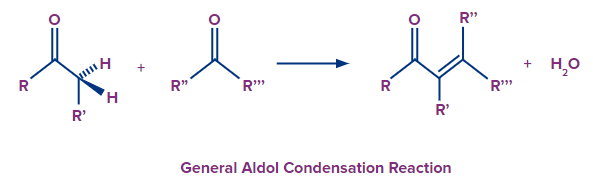
Q 2. What distinguishes the Hofmann rearrangement from the Curtius rearrangement?
- Both reactions have different product
- Both reactions have different reactant
- Both form different isomers
- Both reactions involve different intermediates.
Answer: (B)
Solution: In case of Hofmann rearrangement, A primary amide undergoes the rearrangement when it is treated in water with bromine and the hydroxide ion, resulting in the formation of an amine in which the carbonyl group of the original amide has been removed.
Whereas in case of Curtius rearrangement reaction, an acyl azide is heated in Curtius' reaction, losing nitrogen before rearranging to become an isocyanate.
Hence, both reactions have different reactants, Hoffmann rearrangement is using an amide whereas an acyl azide is taken as reactant in Curtius rearrangement.
Q3. A cyclic diketone undergoes benzylic acid rearrangement, which results in the following:
- Ring fusion
- Alkyl shift
- Ring expansion
- Ring contraction
Answer: (D)
Solution: In the presence of a strong base, 1,2-diketones undergo a rearrangement that results in -hydroxycarboxylic acids. When the subject diketones lack enolizable protons, the highest yields are achieved. An intriguing ring contraction results from the reaction of a cyclic diketone:
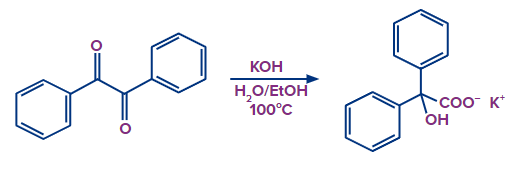
Q.4. Which isomer types are created during rearrangement reactions?
- Tautomers
- Ring chain isomers
- Structural isomers
- Optical isomers
Answer: (C)
Solution: Although the end products have the same molecular formula, their atoms are arranged differently or are bound differently. For instance, the chemical formula of butane and isobutane are identical because they each have the same number of carbon (C) and hydrogen (H) atoms.
Frequently asked questions
Q.1 Why only carbocations show rearrangement reaction, why not free radicals or carbanions?
Answer: Such processes would require higher energy transition states since thermal 1,2 shifts with retention at the migrating centre are prohibited in carbanions by orbital symmetry and a 1,2 shift with inversion at the migrating centre is geometrically challenging. Due to the higher energy, thermal 1,2 shifts involving carbocations are more frequent than the orbital symmetry permitted 1,2 shifts in carbanion systems. Between these two scenarios, a radical process might be realistically anticipated, and once more the transition state would be more energetic (because orbital symmetry is not permitted), making such rearrangements less frequent.
Although less frequent than carbocation shifts, radical and carbanion shifts do happen. High temperatures or weak bonds are necessary in the case of radicals. When the carbanion is stabilised, carbanionic rearrangements are more frequent. This is possible by situating the anion on a sp2or sp hybridised carbon rather than an sp3 hybridised carbon, or by attaching an electronegative heteroatom to the carbanionic centre.
Q.2 How can you determine whether a carbocation will undergo a rearrangement reaction or not?
Answer: A 1,2- hydride shift should happen if a secondary carbocation is close to a tertiary carbon that contains a hydrogen. A 1,2-alkyl shift should happen if a secondary carbocation is close to a quaternary carbon.
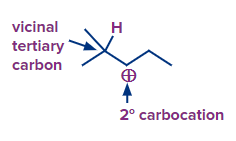
Q.3 Does rearrangement of carbocation undergoes SN2 mechanism?
Answer: Rearrangements occur when a hydride or methyl shift causes a change in the connectivity of the molecule. You must be reminded of the stability of carbocations in order to explain why and how this occurs: Alkyl groups' ability to donate electrons and the hyperconjugation process make substituted carbocations more stable.
Since SN2and E2 reactions are coordinated mechanisms in which everything occurs at once, carbocations can only be created in unimolecular (SN1 and E1) reactions.
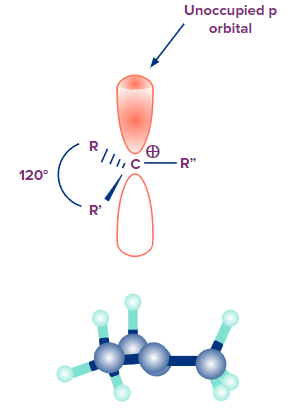
Q.4 What is the geometry of carbocation?
Answer: The carbon sp2 becomes hybridised when a carbocation has three substituents, which results in a trigonal planar shape for the entire molecule. The bond angle between the substituents of the carbocation is 120°, and they are all located in the same plane. It only has six valence electrons, which are required to create three sigma covalent bonds with the substituents, hence the carbon atom in the carbocation is electron deficient. The unoccupied p orbital of the carbocation carbon is parallel to the plane made by the substituents. Carbocations make excellent Lewis acids because the p orbital can readily receive electron pairs during reactions.