-
Call Now
1800-102-2727
Electrophilic Aromatic Substitution Reactions of Benzene – Mechanism and Types of Electrophilic Aromatic Substitution Reactions of Benzene
At some point in your life, you must have had a bicycle flat tyre. This predicament, particularly in the middle of the road, can occasionally become extremely frightening, but it gets worse when it is not solved even after numerous tries.
All we can do in this situation is swap out the old tyre with a new one. The old tyre cannot be simply replaced with any other random tyre. You need to confirm that aspects like tyre quality, height, and width correspond to the sort of bicycle you ride. Therefore, the new tyre essentially replaces the old one.
Similar chemical reactions exist in chemistry and they have a lot in common with changing a flat tyre. Depending on the situation, a functional group can replace the present functional group on the benzene ring.
In this article, we will learn more about the electrophilic aromatic substitution reactions of benzene in detail.
TABLE OF CONTENTS
- Electrophilic Aromatic Substitution Reactions of Benzene
- Electrophilic Aromatic Substitution Reactions of Benzene – General Mechanism
- Electrophilic Aromatic Substitution Reactions of Benzene – Types
- Practice Problems
- Frequently Asked Questions – FAQ
Electrophilic Aromatic Substitution Reactions of Benzene
Electrophilic aromatic substitution reactions are organic reactions in which an atom connected to an aromatic ring is replaced by an electrophile. In these reactions, an electrophile normally takes the place of a hydrogen atom from a benzene ring, i.e an electrophile substitutes a hydrogen atom from the benzene ring. The aromaticity of the aromatic system is preserved by an electrophilic aromatic substitution process. For instance, the stability of the aromatic ring is not compromised when bromobenzene is created when benzene and bromine combine. The graphic below shows this response.
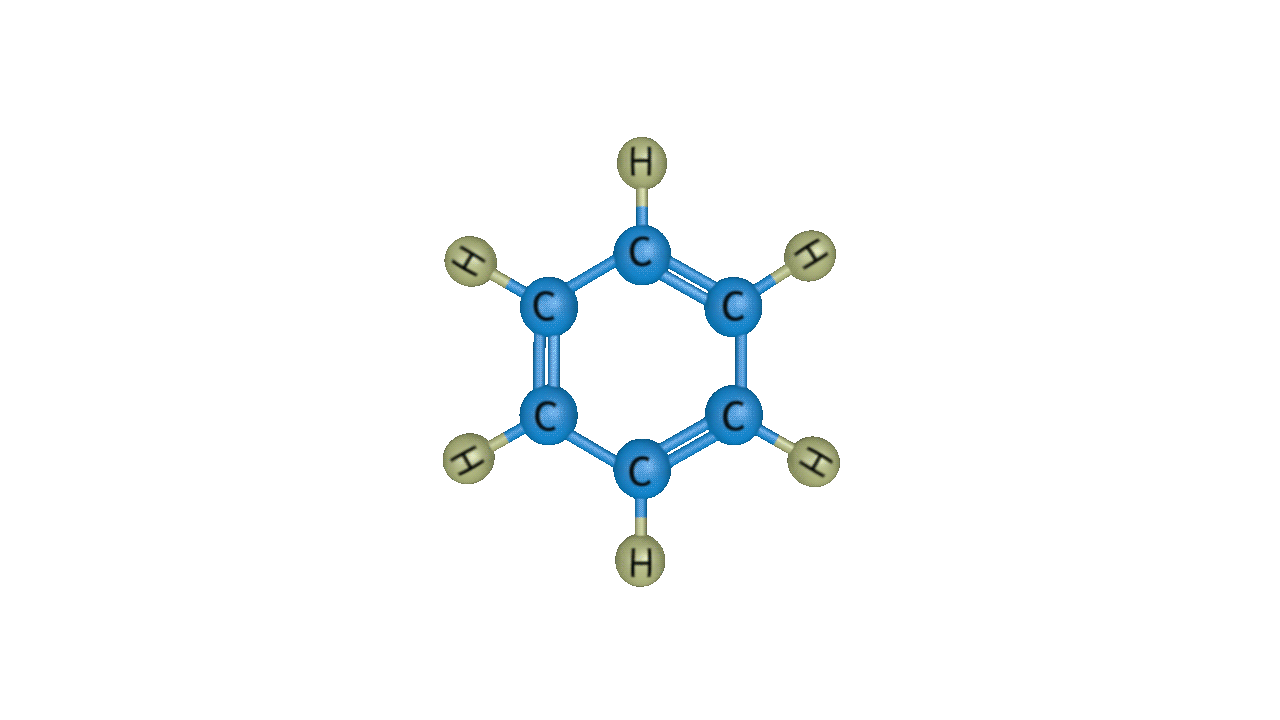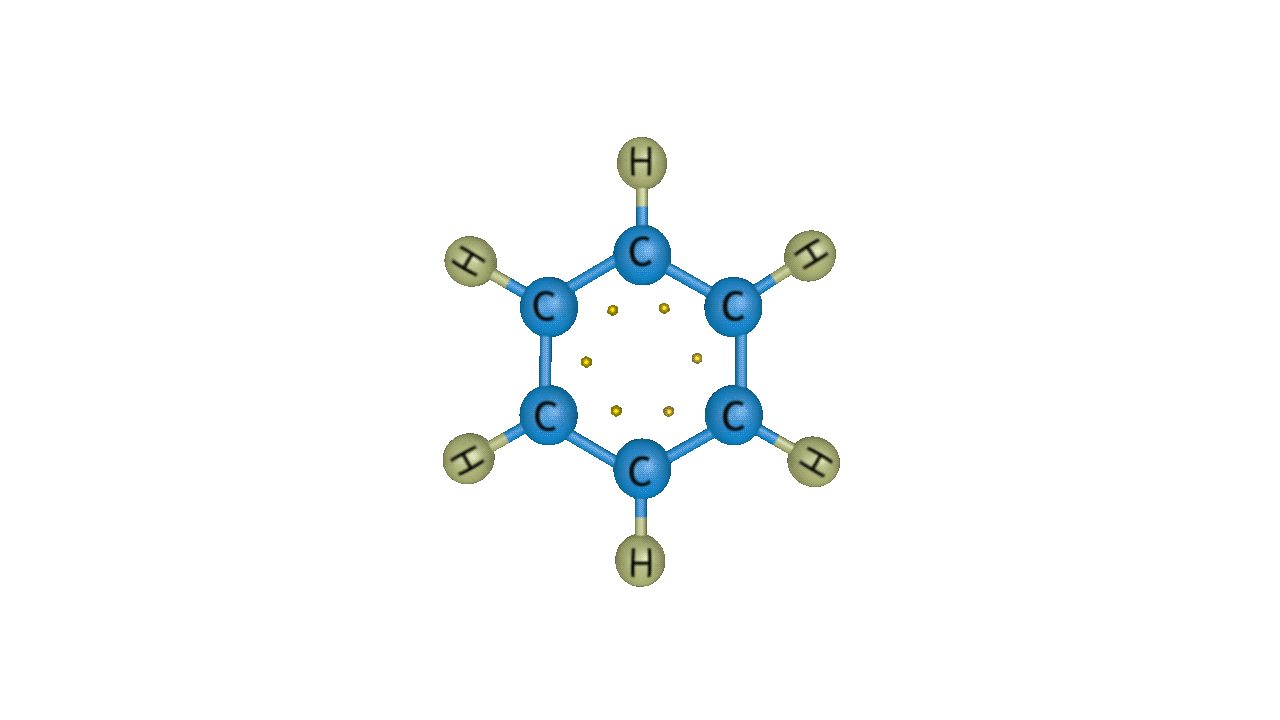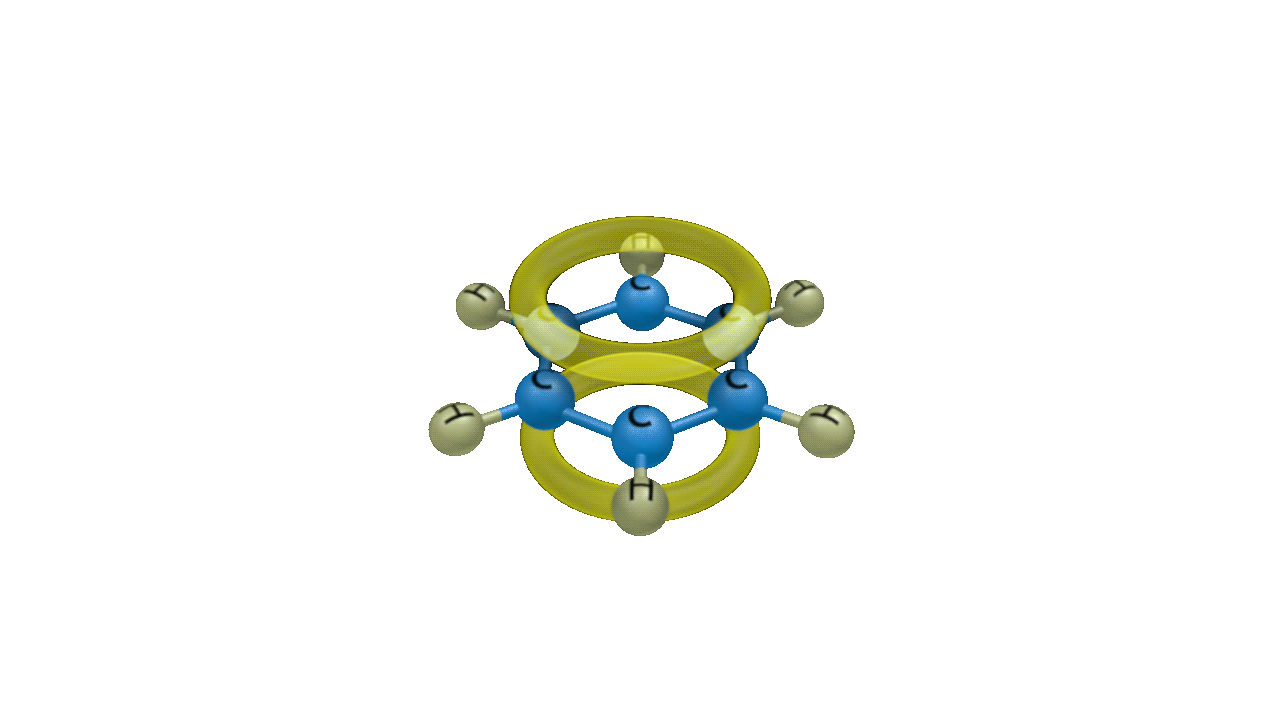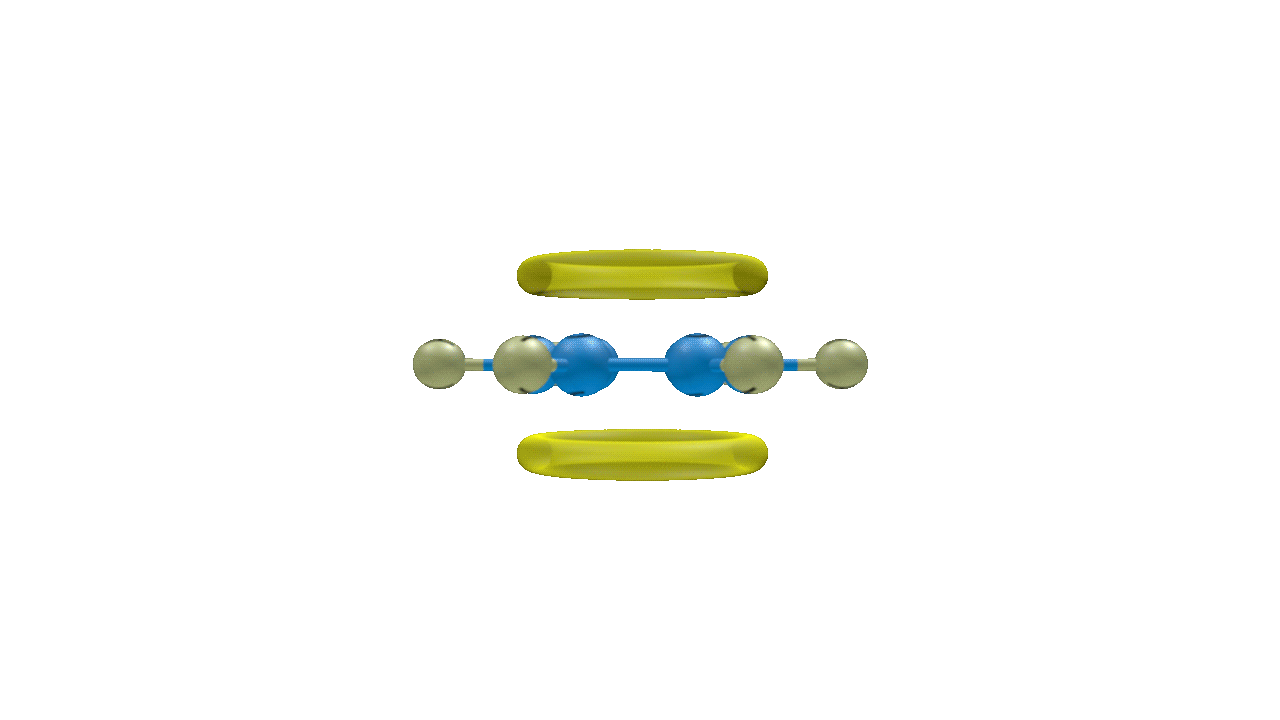
Though benzene is susceptible to electrophilic attack, it generally undergoes substitution reactions rather than addition reactions. Because benzene is an electron-rich system with delocalized -electrons, it undergoes electrophilic substitution reactions. Electrophilic substitution of benzene involves the reaction of an electrophile with an aromatic compound. Therefore, it is more precisely called as Electrophilic Aromatic Substitution Reaction.
The generalised reaction of EAS (Electrophilic Aromatic Substitution) of benzene can be represented as:
Electrophilic Aromatic Substitution Reactions of Benzene – General Mechanism
Step 1: Generation of an Electrophile
The creation of an electrophile requires the usage of a Lewis acid. An excellent Lewis acid for producing electrophiles for electrophilic aromatic substitution is anhydrous aluminium chloride. The electrophiles that are produced when anhydrous aluminium chloride and the attacking agent are combined and can be used in the electrophilic aromatic substitution processes are illustrated below.
|
Electrophile (E+) |
Name |
Source |
Name of the reaction |
|
Cl+ |
Chloronium |
Cl2+AlCl3 |
Chlorination |
|
Br+ |
Bromonium |
Br2+AlBr3 |
Bromination |
|
NO2+ |
Nitronium |
HNO3+H2SO4 |
Nitration |
|
SO3 |
Sulphur trioxide |
Fuming H2SO4 |
Sulphonation |
|
R+ |
Alkyl Carbocation |
RX+AlX3 X = Cl, Br |
Friedel-Crafts Alkylation |
|
R-C+=O |
Acyl Carbocation |
RCOCl+AlCl3 |
Friedel-Crafts Acylation |
Step 2: Formation of Arenium ion (Carbocation)
In the second step, the electrophile formed in the first step will attack on the benzene ring to form an intermediate carbocation which is called as -complex or arenium ion or Wheland intermediate.
The formed arenium ion is resonance stabilised. The resonance forms of arenium ions are:
![]()
This is the slowest step, and therefore determines the rate of the electrophilic aromatic substitution process. Since two molecules are involved in the step that determines the rate, electrophilic aromatic substitution processes are bimolecular.
Step 3: Removal of Proton
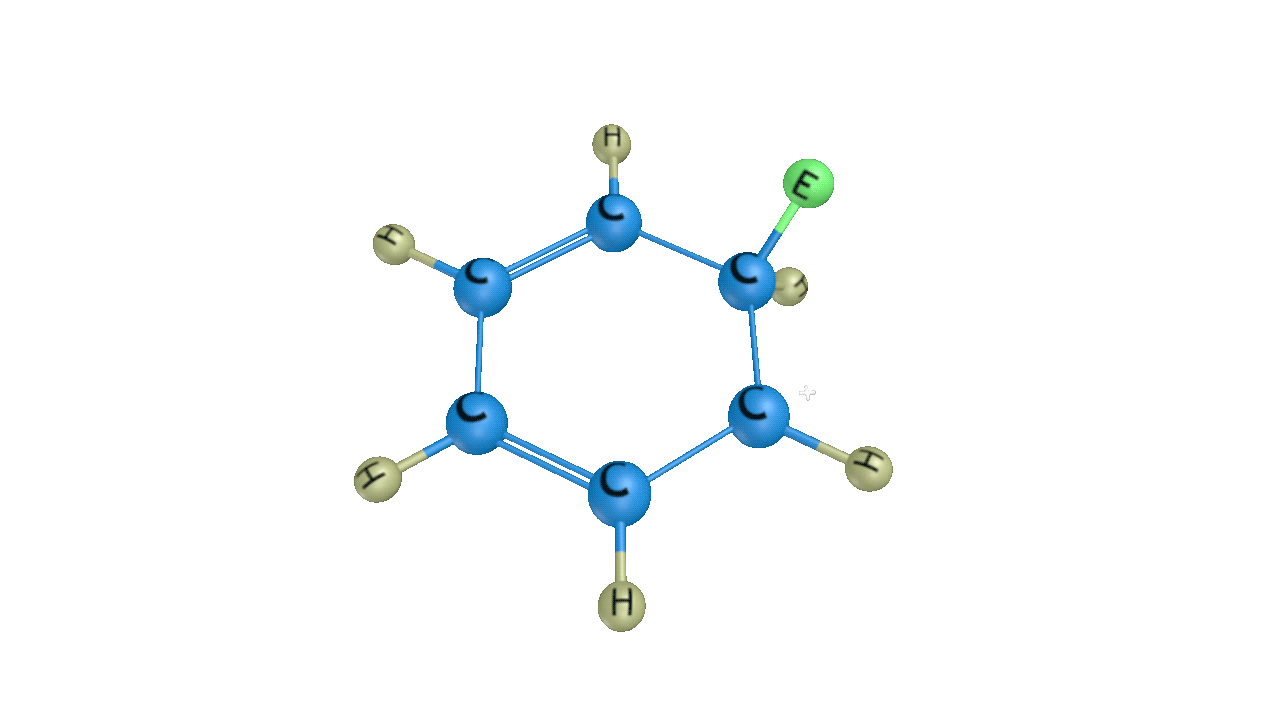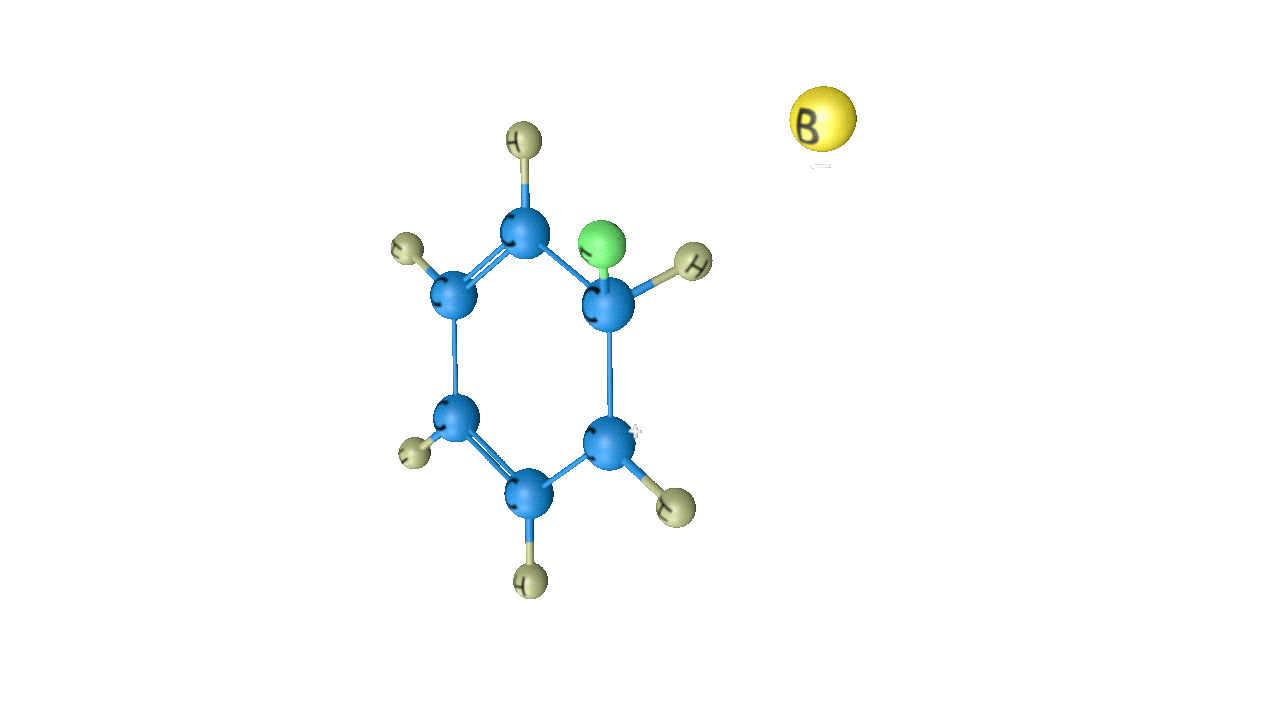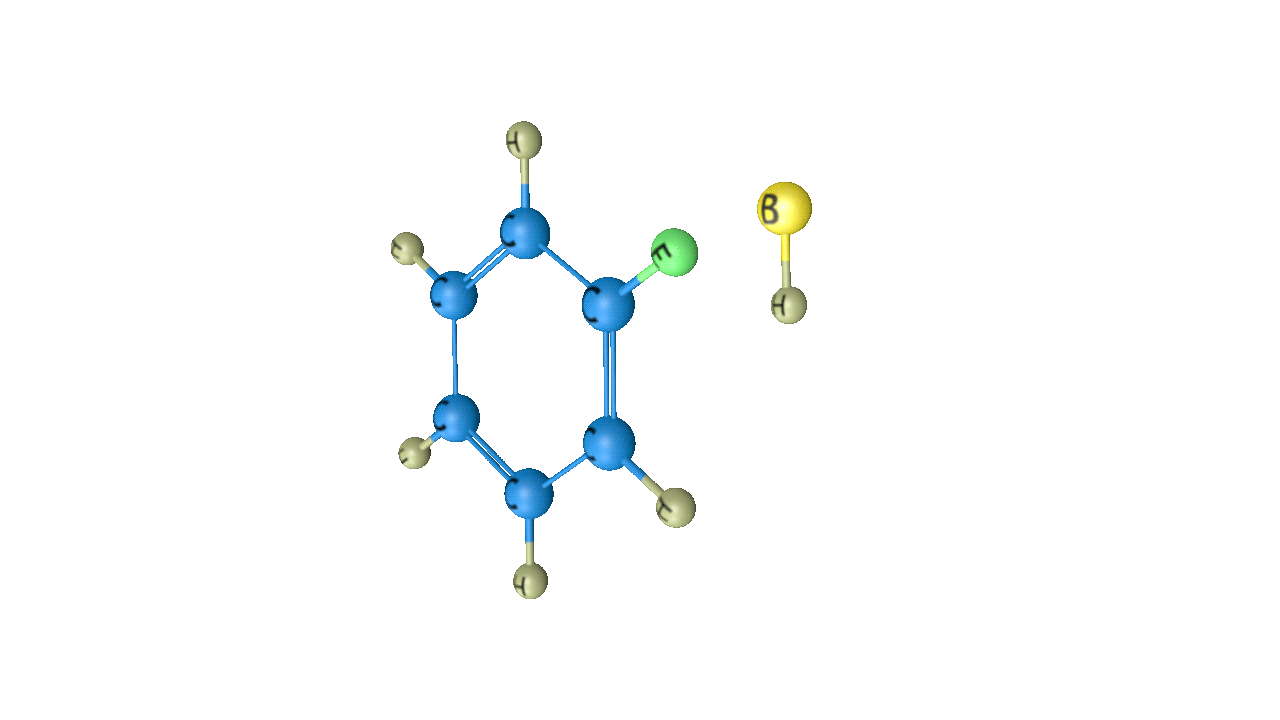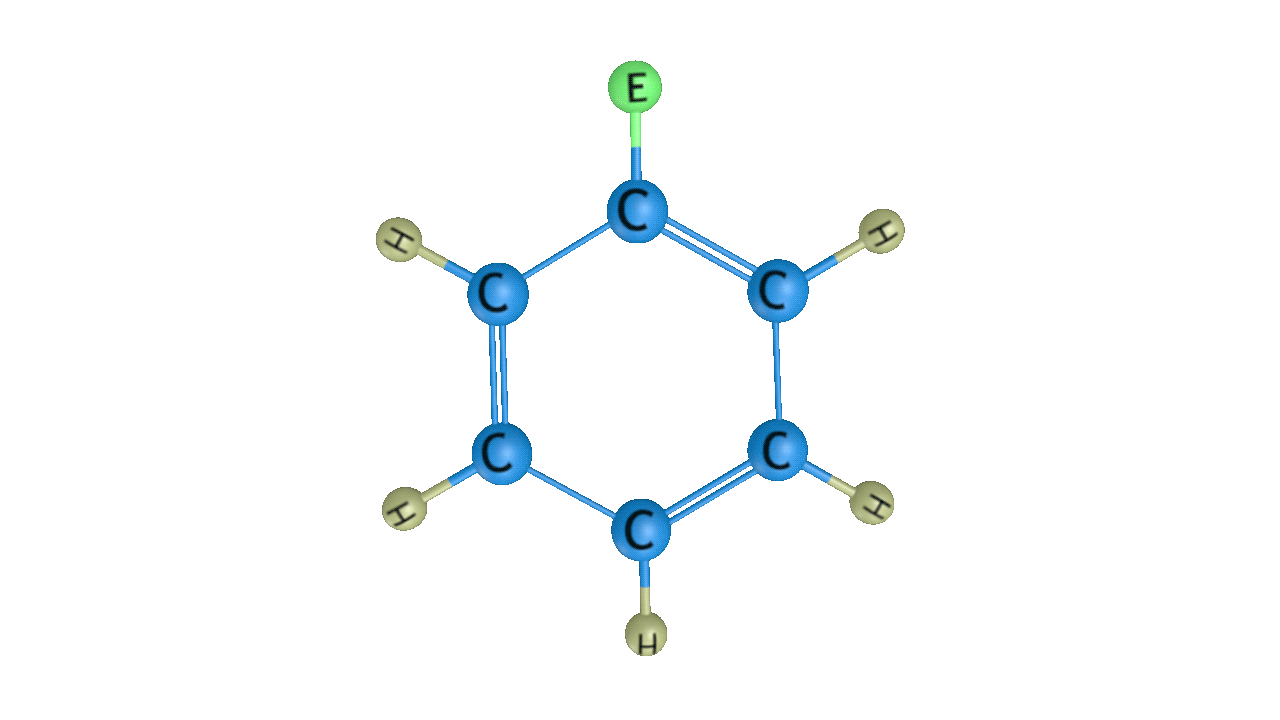
When this sigma complex is attacked by the base, the sigma complex releases a proton from the sp3 hybridised carbon in order to restore the aromatic character, and as a result substituted product is formed. The following reaction describes the removal of a proton from this particular sigma complex.
As a result, the hydrogen atom in the benzene ring is replaced by the electrophile. It is a crucial reaction in organic chemistry since the idea of electrophilic substitution is used in many chemical name reactions.
Electrophilic Aromatic Substitution Reactions of Benzene – Types
Here is a list of most commonly encountered electrophilic aromatic substitution reactions of benzene.
- Friedel-Crafts Alkylation
- Friedel-Crafts Acylation
- Electrophilic Aromatic Halogenation Reaction
- Electrophilic Aromatic Nitration Reaction
- Electrophilic Aromatic Sulfonation Reaction
- Electrophilic Aromatic Mercuration Reaction
- Formylation Reaction
- Gattermann Aldehyde Reaction
- Blanc Reaction
Friedel-Crafts Alkylation
Friedel-Crafts alkylation is a classic chemical reaction where the proton (H+) in the aromatic compound gets substituted with the alkyl group. The reaction takes place in the presence of a catalyst which is anhydrous aluminium chloride. Anhydrous AlCl3 may also be replaced with any other Lewis acids such as ferric chloride (FeCl3).
Mechanism of Friedel-Crafts Alkylation
The mechanism of the Friedel-Crafts alkylation reaction may be illustrated in the steps given below.
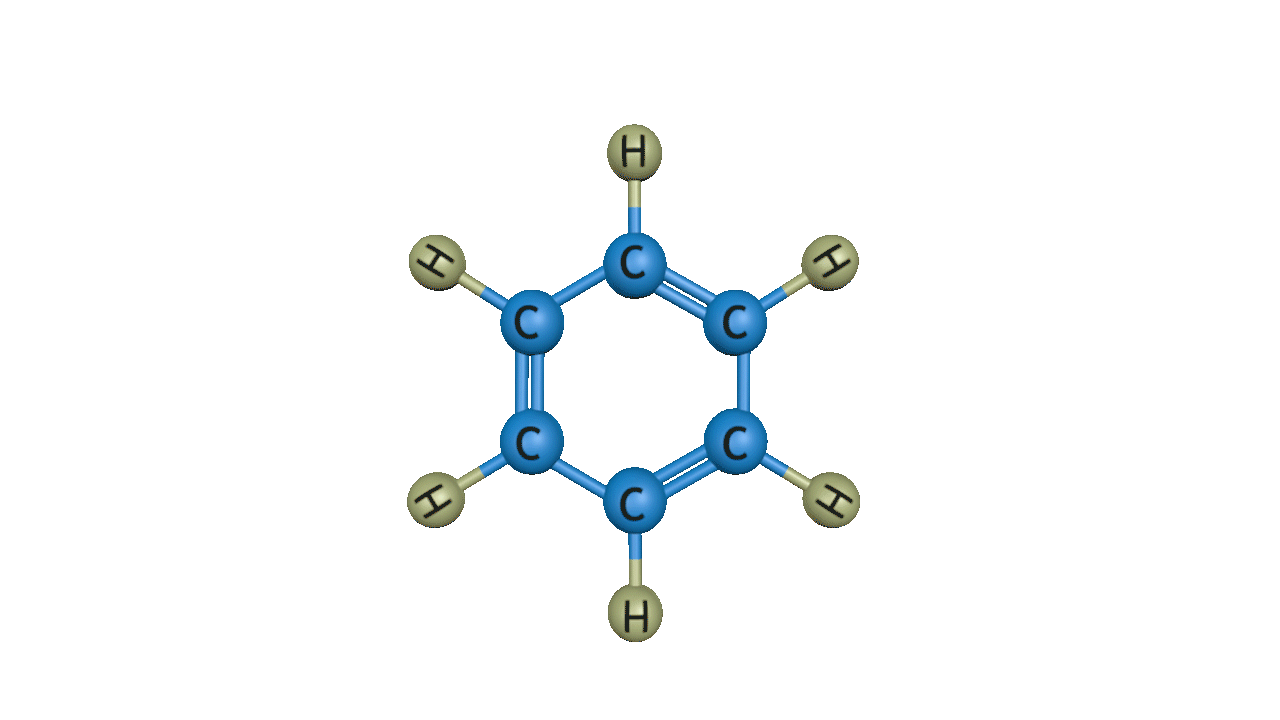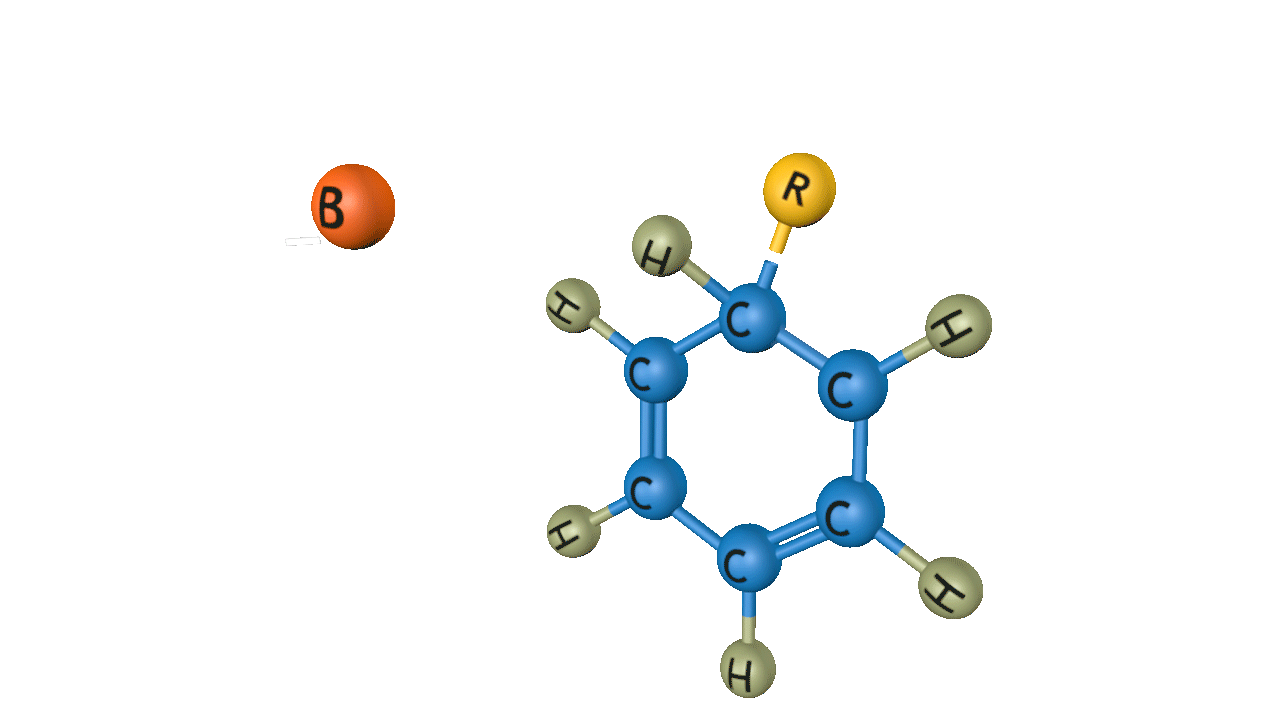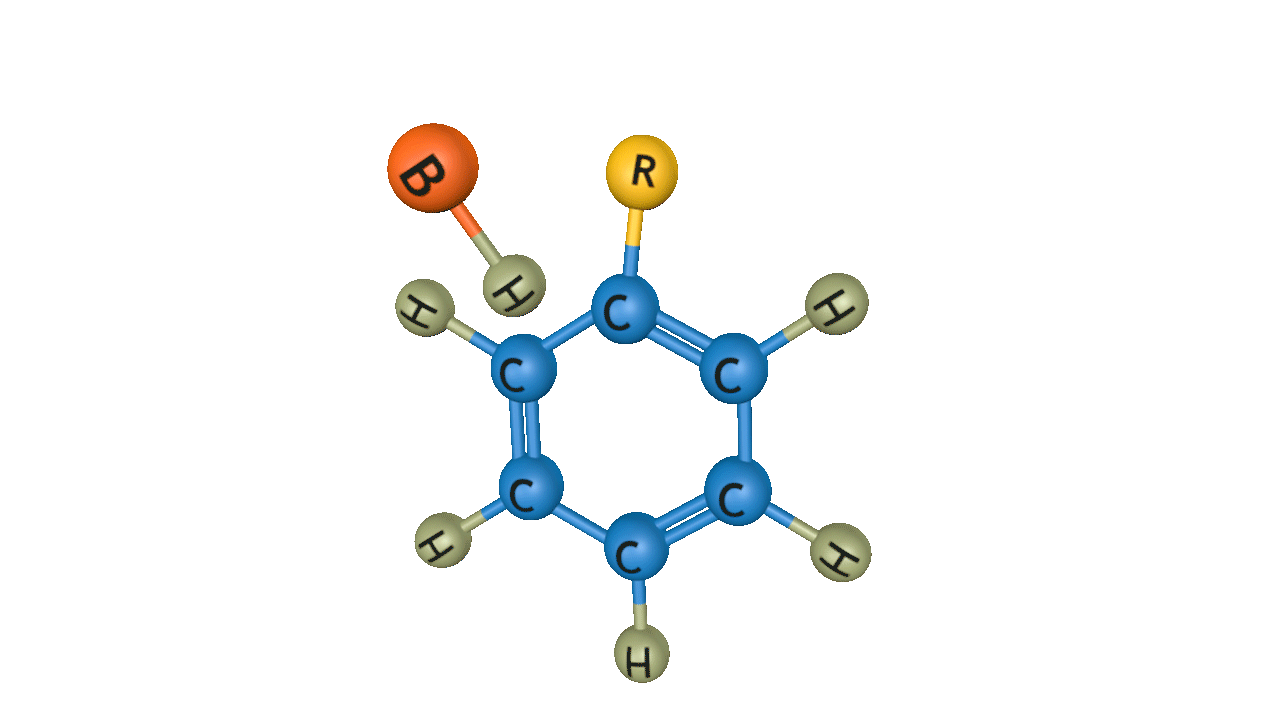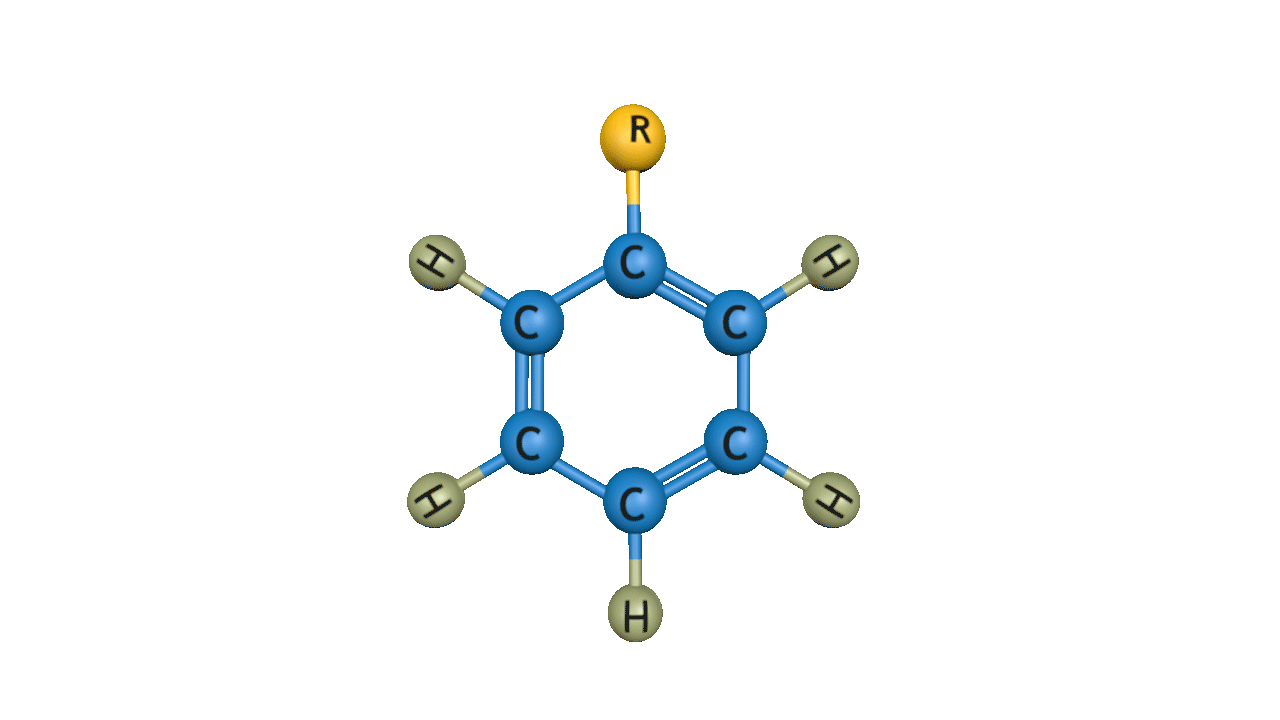
Step 1: Generation of the electrophile
Anhydrous aluminium chloride or ferric chloride is used as the Lewis acid in the process, which involves the alkyl halide. Typically, an electrophilic carbocation results from this process.

Step 2: Formation of an intermediate
The electrophilic carbocation formed when Lewis acid and alkyl halide combine attacks the aromatic ring. After attacking the aromatic ring, cyclohexadienyl cation (𝜎- complex) is the intermediate that results. The carbon-carbon double bond breaks, which causes the aromatic ring to lose its scent.
Step 3: Removal of proton from the arenium ion
The cyclohexadienyl cation goes through deprotonation, or proton loss. The aromatic ring's carbon-carbon double bond is reformated, which also returns the ring's aromaticity. The aluminium chloride catalyst is renewed by the proton released during deprotonation.
Order of rate of Friedel-Crafts Alkylation is
Limitations of Friedel-Crafts alkylation
Friedel-craft’s alkylation reaction has the following limitations.
- Vinyl and aryl halides do not generate stable carbocations. They are therefore ineligible for use in the Friedel-Crafts alkylation procedure.
- Deactivating groups remove the electron density from the benzene ring, making an electrophilic attack on the ring impossible, hence aromatic rings containing these groups may not be suited for Friedel-Crafts alkylation.
- Anhydrous aluminium chloride and aniline interact to generate a compound that renders the ring inactive. This leaves the reaction unfinished.
- The benzene ring undergoes alkylation when it combines with an alkyl halide in the presence of anhydrous Lewis acid, however polyalkylation may also result due to the alkyl group's activating nature. The aromatic sample needs to be administered in big doses to prevent this effect.
- Due to their low reactivity, substances like mono halobenzenes do not react or take part in the Friedel-Crafts alkylation reaction.
Friedel-Crafts Acylation
The Friedel-Crafts alkylation reaction and the Friedel-Crafts acylation reaction are comparable. The main distinction is that, in contrast to an alkylation reaction, the Friedel-Crafts acylation reaction results in the creation of a ketone.
Different aromatic compounds can be used with the Friedel-Crafts acylation process. However, the oxygen and nitrogen atoms go through acylation if the reactant is alcohol or amine, respectively.
Mechanism of Friedel-Crafts Alkylation
The mechanism of the Friedel-Crafts alkylation reaction may be illustrated in the steps given below.
Step 1: Generation of the electrophile
An acylium ion is created when the acyl halide and anhydrous aluminium chloride react. Resonance is used to stabilise the generated acylium ion..

Step 2: Formation of an intermediate
The aromatic ring is attacked by the acylium ion that is created when Lewis acid and acyl halide combine. An intermediate is created after it attacks the aromatic ring. The carbon-carbon double bond breaks, which results in the ring losing its aromaticity.
Step 3: Removal of proton from the arenium ion
The intermediate complex proceeds through deprotonation, or proton loss. The aromatic ring's carbon-carbon double bond is reformated, which also returns the ring's aromaticity. The aluminium chloride catalyst is renewed by the proton released during deprotonation.
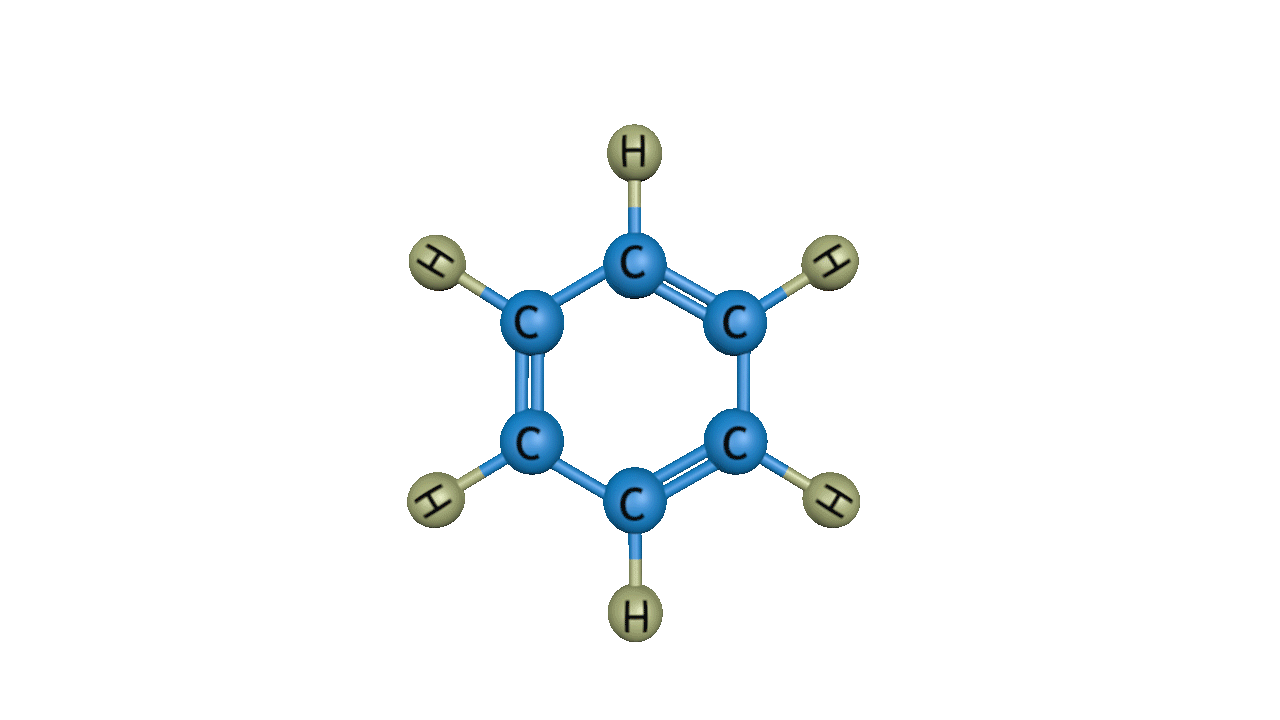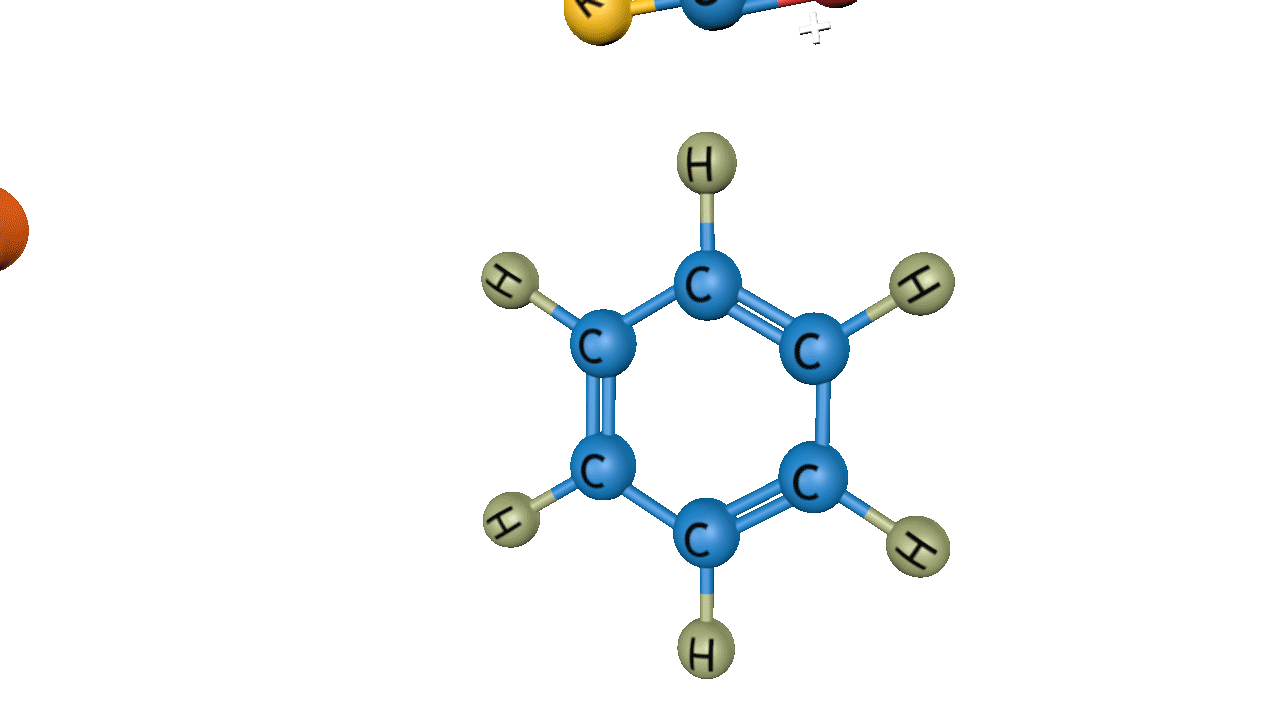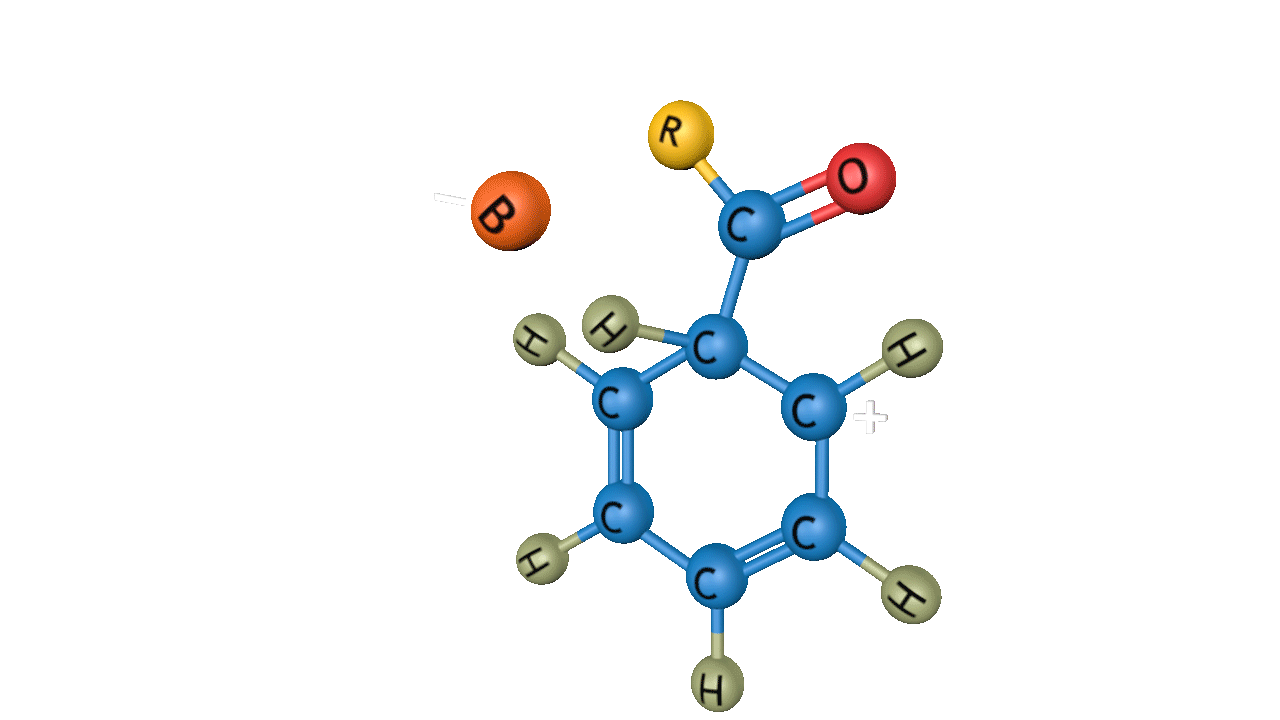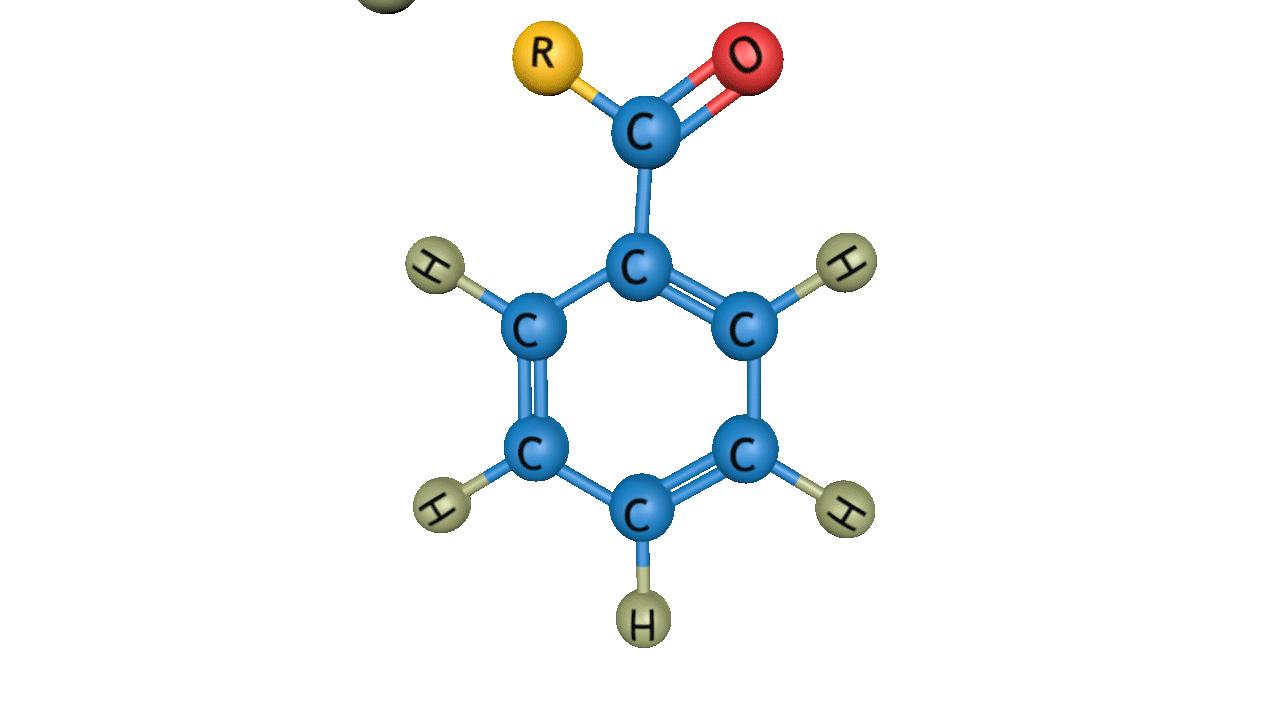
The general reactivity order of Friedel-Crafts acylation reaction is
Limitation of Friedel-Crafts Acylation reaction
Friedel-craft’s acylation has the following limitations
- Only ketone compounds are produced as a result of the Friedel-Crafts acylation process.
- Due to their low reactivity, substances like mono halobenzenes do not react or take part in the Friedel-Crafts acylation procedure.
- Aryl amines are also not suitable for Friedel-Crafts acylation reaction. Because, lewis acid catalyst reaction causes aryl amines to produce unreactive complexes, which makes them unsuitable for Friedel-Crafts acylation process.
Electrophilic Aromatic Halogenation Reaction
Benzene reacts with bromine or chlorine in the presence of Lewis acids to give the corresponding halogenated substitution products in good yield. Lewis acids typically used are AlCl3 or FeCl3 for chlorination, and FeBr3 for bromination.
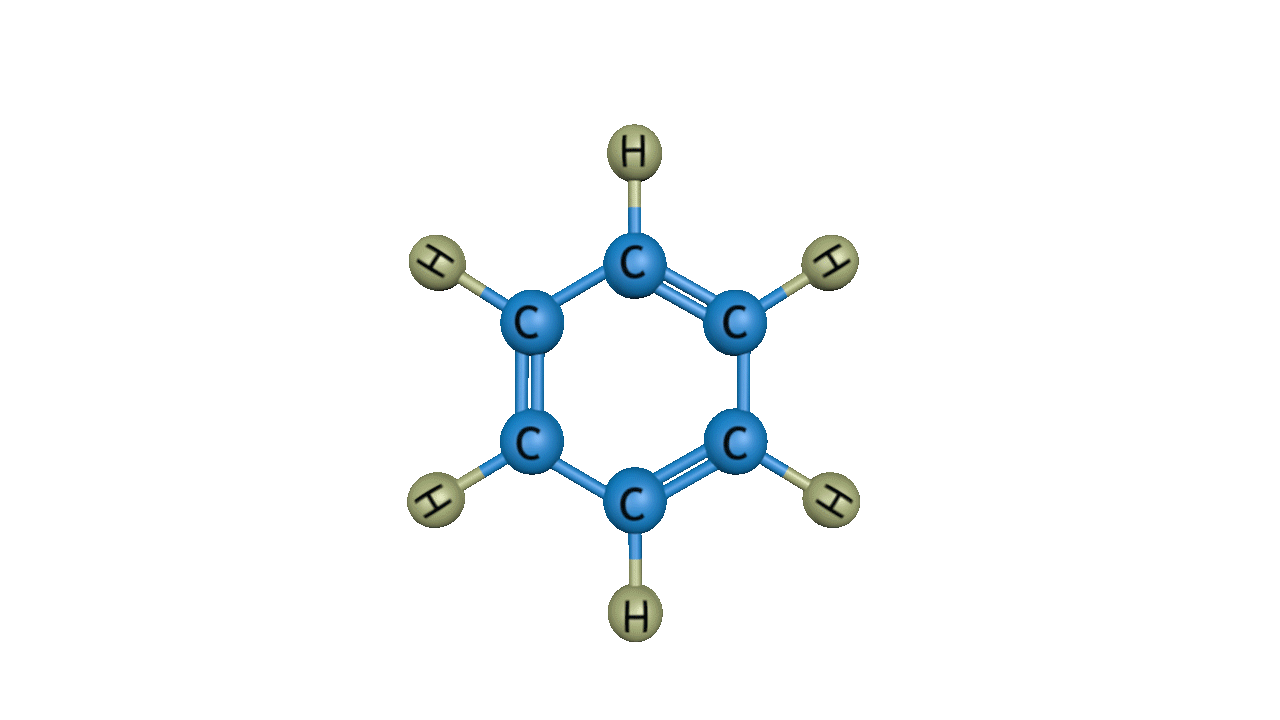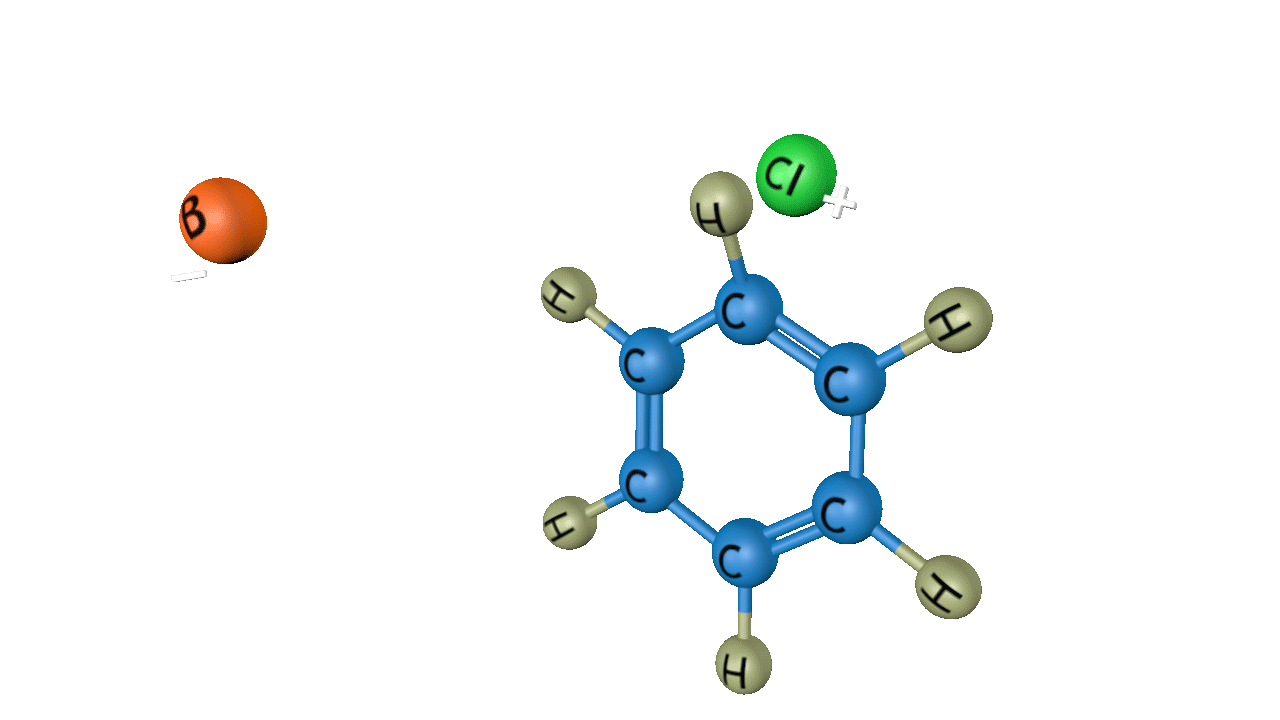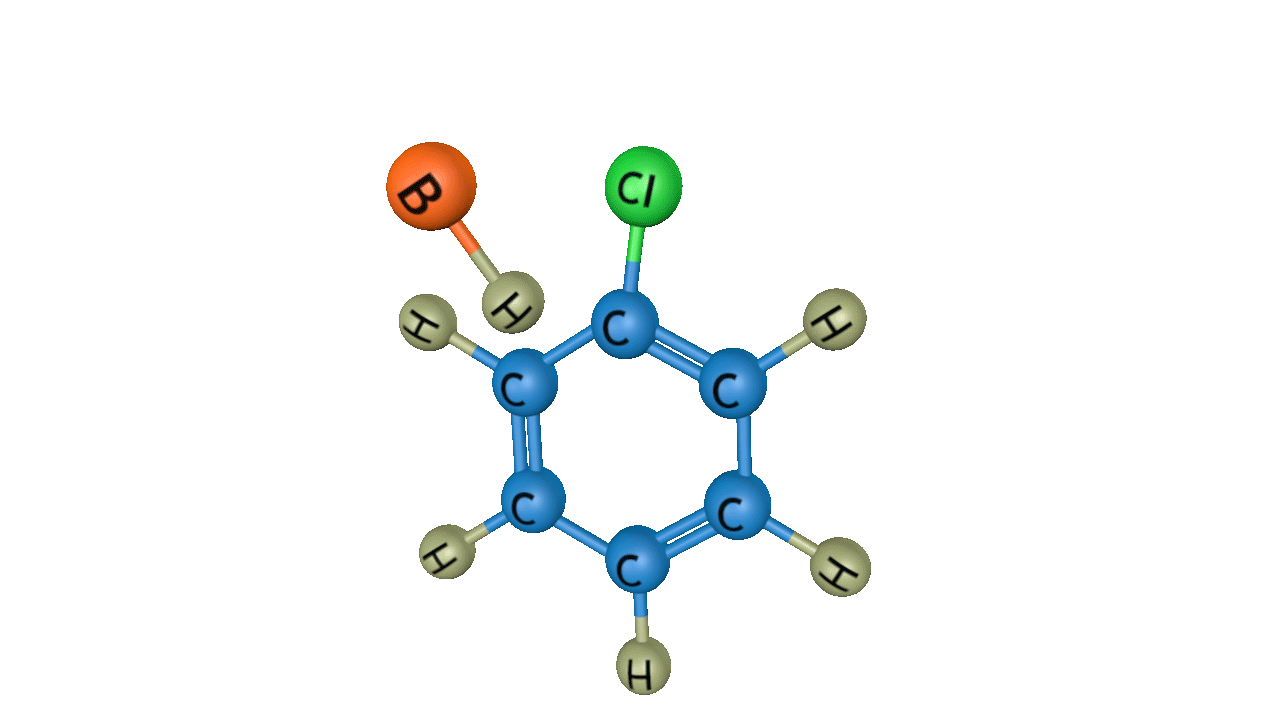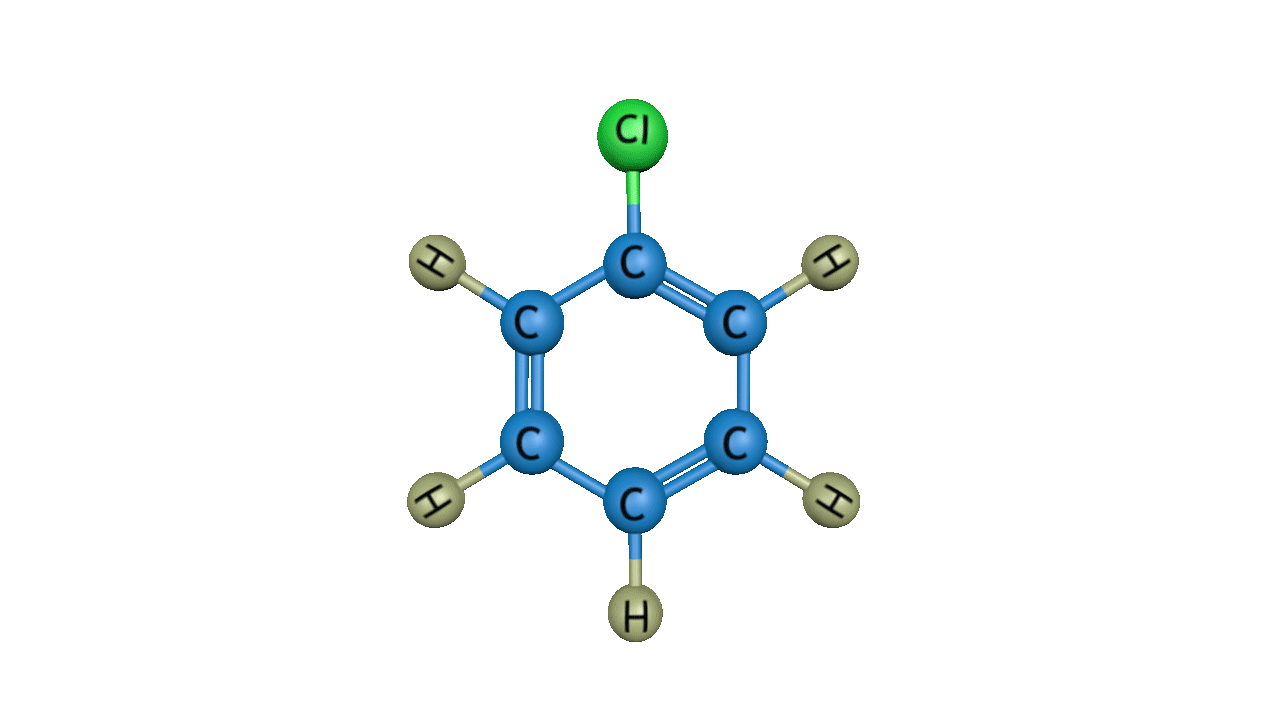
Chlorination
Benzene undergoes chlorination when it is treated with chlorine in the presence of Lewis catalysts such as AlCl3 or FeCl3 and in the absence of light.
Mechanism of chlorination
Step 1: Generation of the electrophile
Anhydrous aluminium chloride or ferric chloride is used as the Lewis acid in the process, which involves Cl2. Typically, a chlorine(I) cation results from this process.

Step 2: Formation of an intermediate
Electrophile (Cl+) is formed in the first step and attacks the benzene ring in order to form an intermediate carbocation which is resonance stabilised. Formation of a carbocation is the slowest and rate-determining step of chlorination reaction.
The resonance forms of carbocation are:
Step 3: Removal of proton from the arenium ion
In the third step, by the attack of a base, the sigma complex releases a proton from the sp3 hybridised carbon in order to restore the aromatic character, and chlorobenzene is formed as a product.
Bromination
Benzene undergoes bromination when it is treated with chlorine in the presence of Lewis catalysts such as FeBr3 and in the absence of light. The bromination of benzene is not as fast as the chlorination of benzene.
Mechanism of bromination:
Step 1: Generation of the electrophile
Anhydrous ferric bromide is used as the Lewis acid in the process, which involves Br2. Typically, a bromine(I) cation results from this process.
Step 2: Formation of an intermediate
The first step results in the formation of an electrophile, which attacks the benzene ring to create an intermediate carbocation that is resonance stabilised. This step is the slowest and hence, is the rate-determining step.
Step 3: Removal of proton from the arenium ion
In the third step, by the attack of nucleophile, the sigma complex releases a proton from the sp3 hybridised carbon in order to restore the aromatic character, and bromobenzene is formed as a product.
Iodination of benzene is a slow as well as reversible process. It occurs in the presence of an oxidising agent i.e., HIO3,HNO3 or HgO to remove formed HI which is a strong reducing agent.
It is impossible to fluorinate benzene because an explosion occurs anytime fluorine combines with benzene. Because of how quickly fluorine interacts with benzene, special equipment and conditions are needed for aromatic fluorination. Even yet, it remains challenging to restrict the reaction to monofluorination.
C6H6 +3F2 6HF +6C
In presence of excess of halogens and anhydrous AlCl3 (catalyst) and dark, all the hydrogen atoms of the benzene ring may be successively substituted.
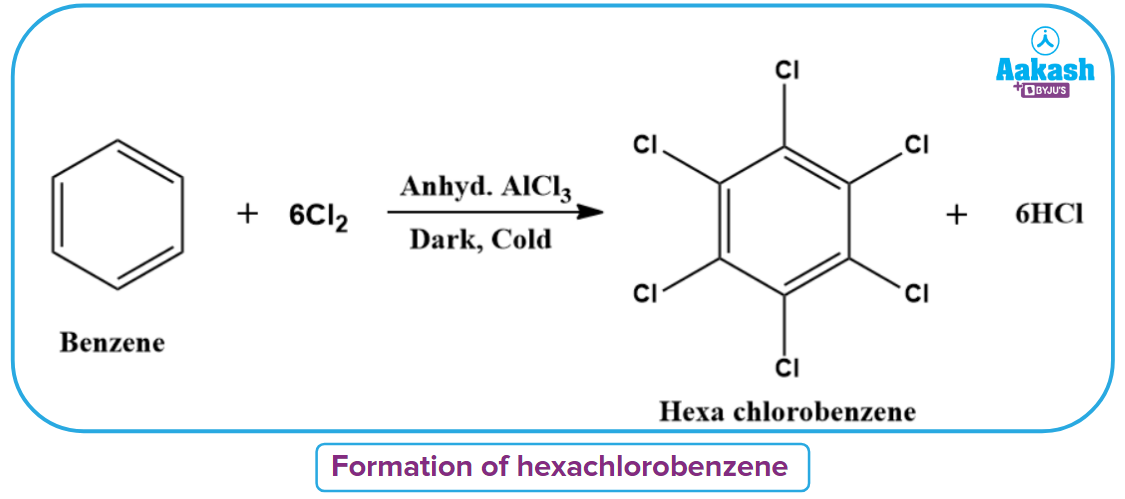
Electrophilic Aromatic Nitration Reaction
Nitrobenzene is produced when concentrated nitric acid and concentrated sulfuric acid are used to treat benzene. A hydrogen atom in the benzene ring is replaced with a nitro group. This reaction is carried out at 40-50C temperature.
Mechanism of nitration
Step 1: Generation of the electrophile
When nitric acid receives a proton from sulfuric acid and then dissociates, the nitronium ion is produced.
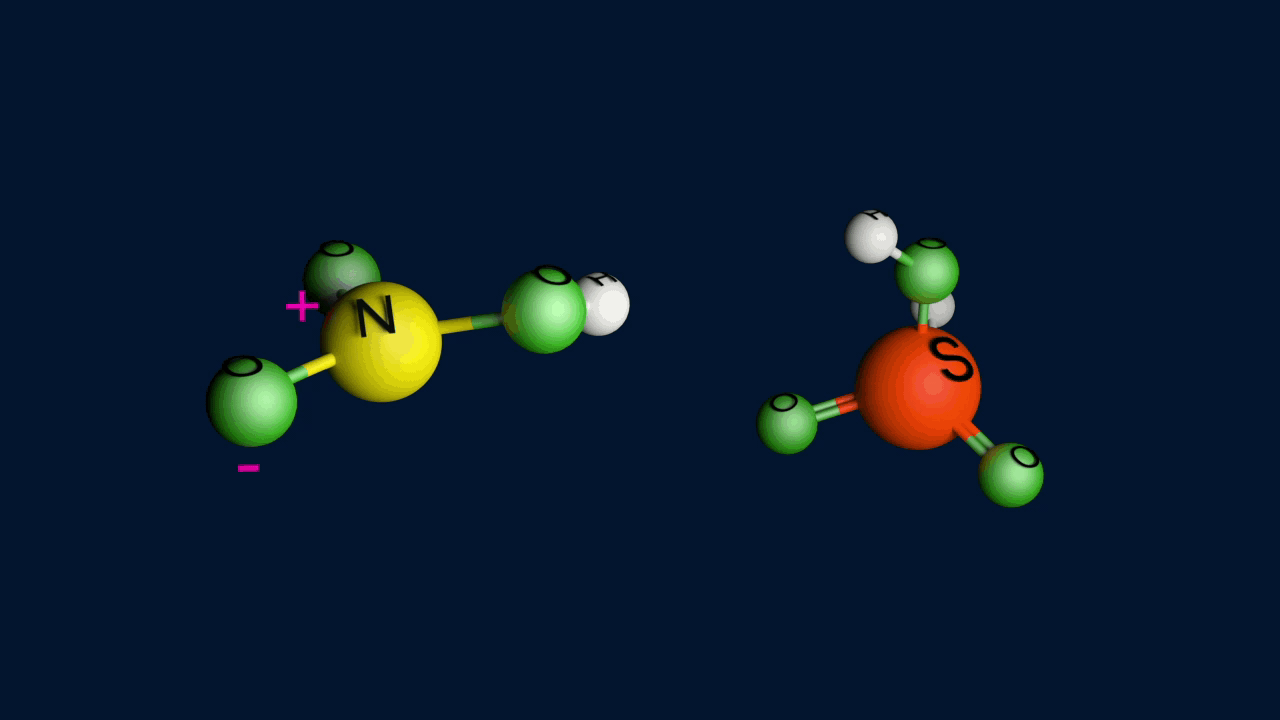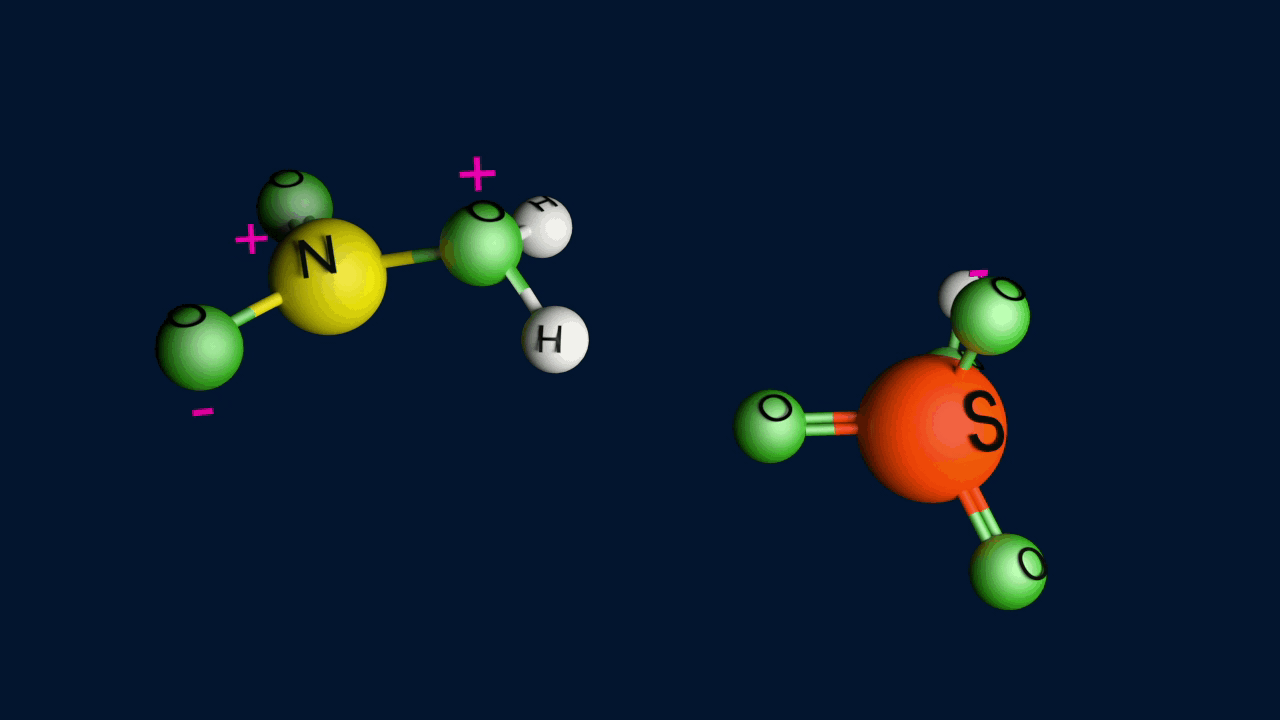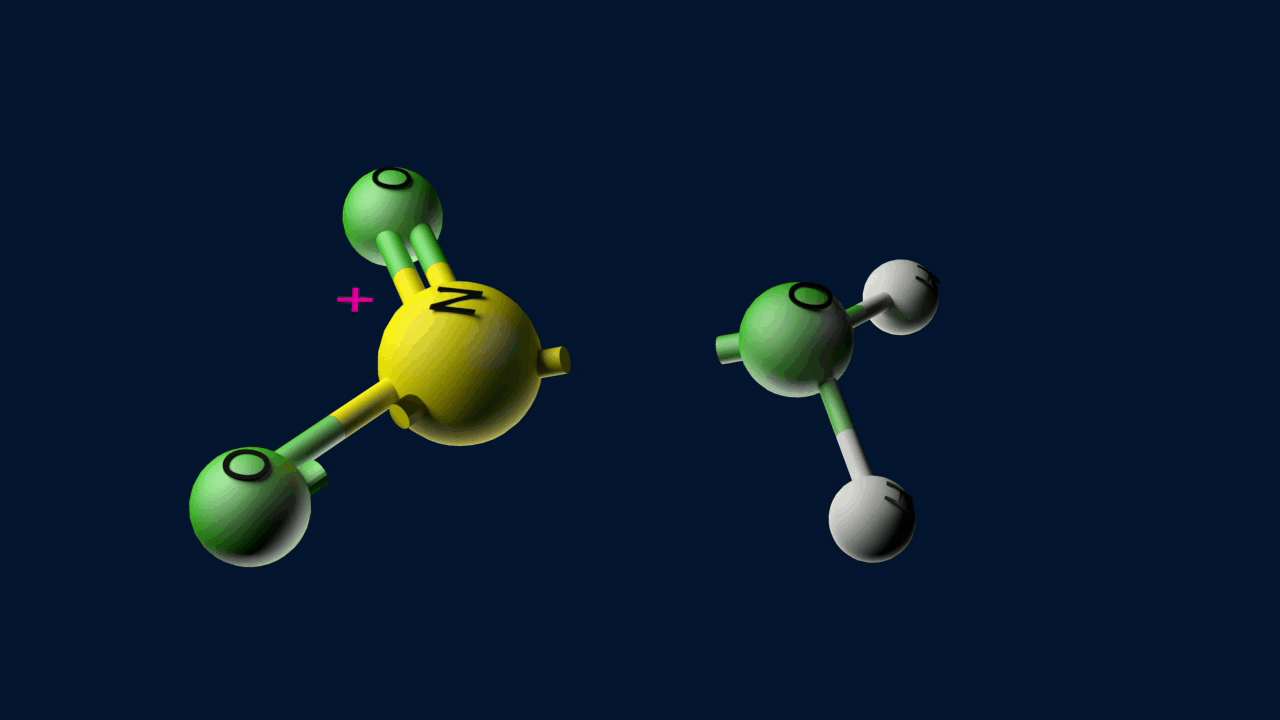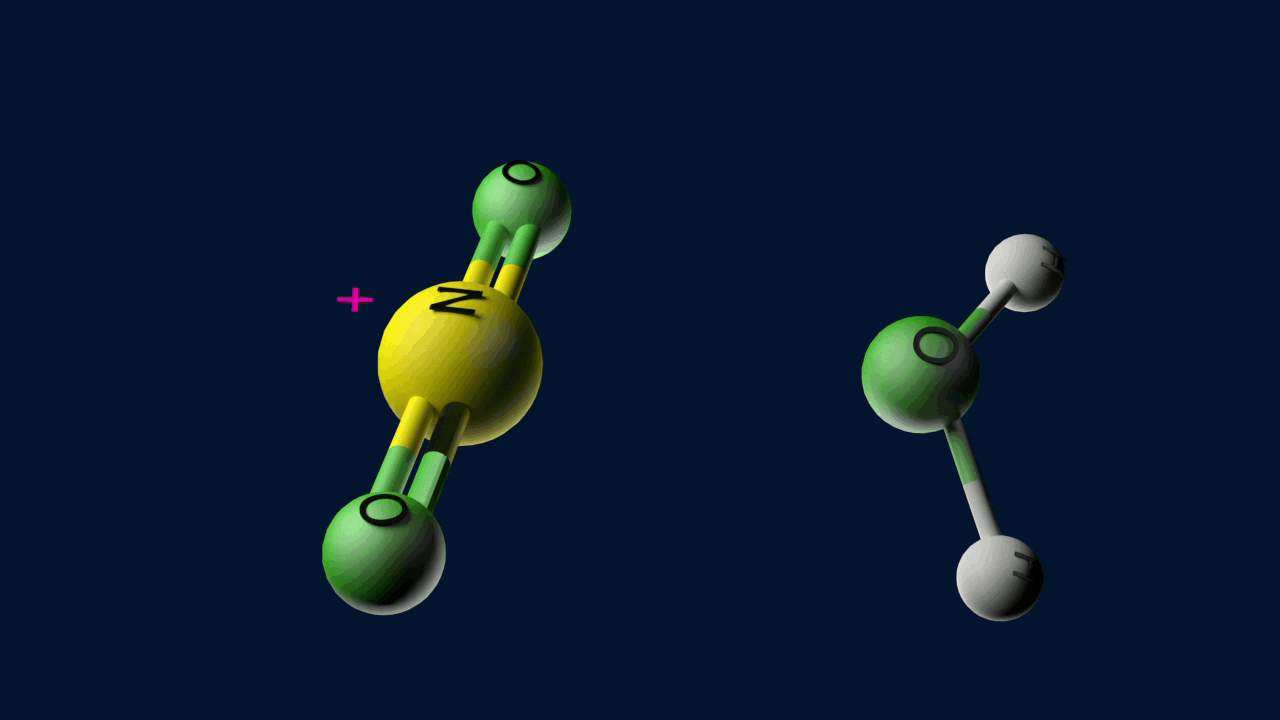
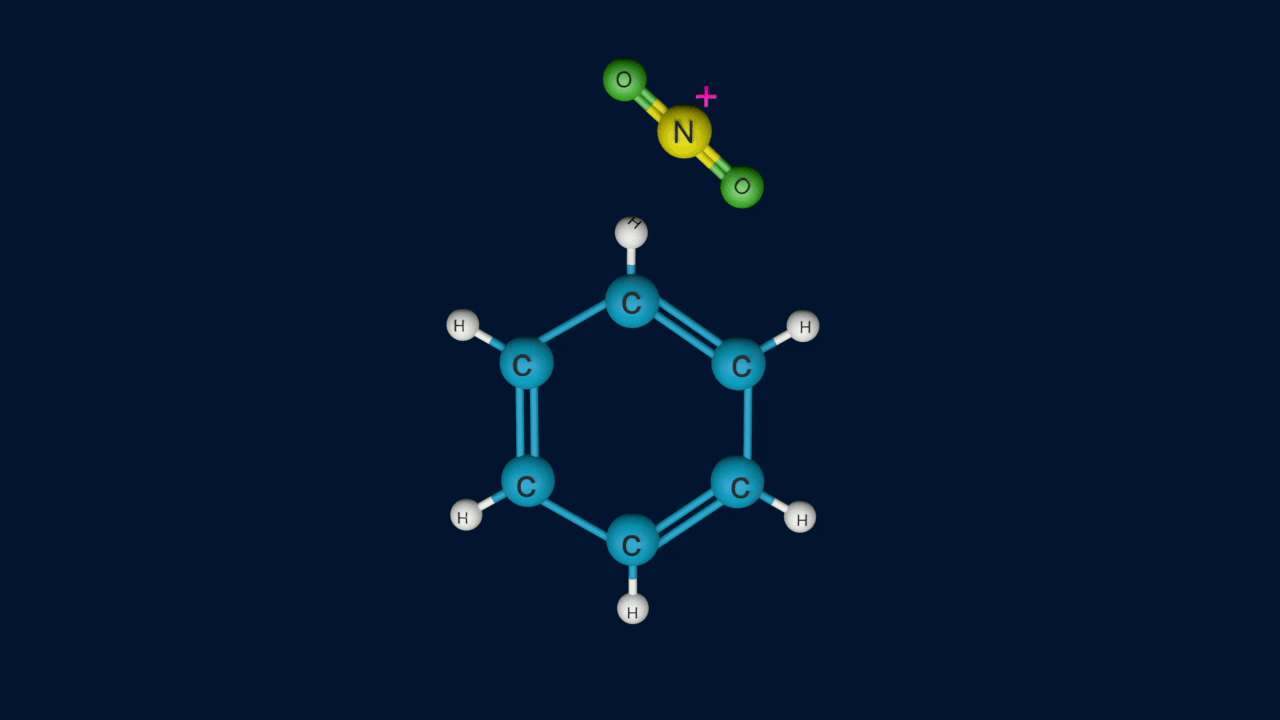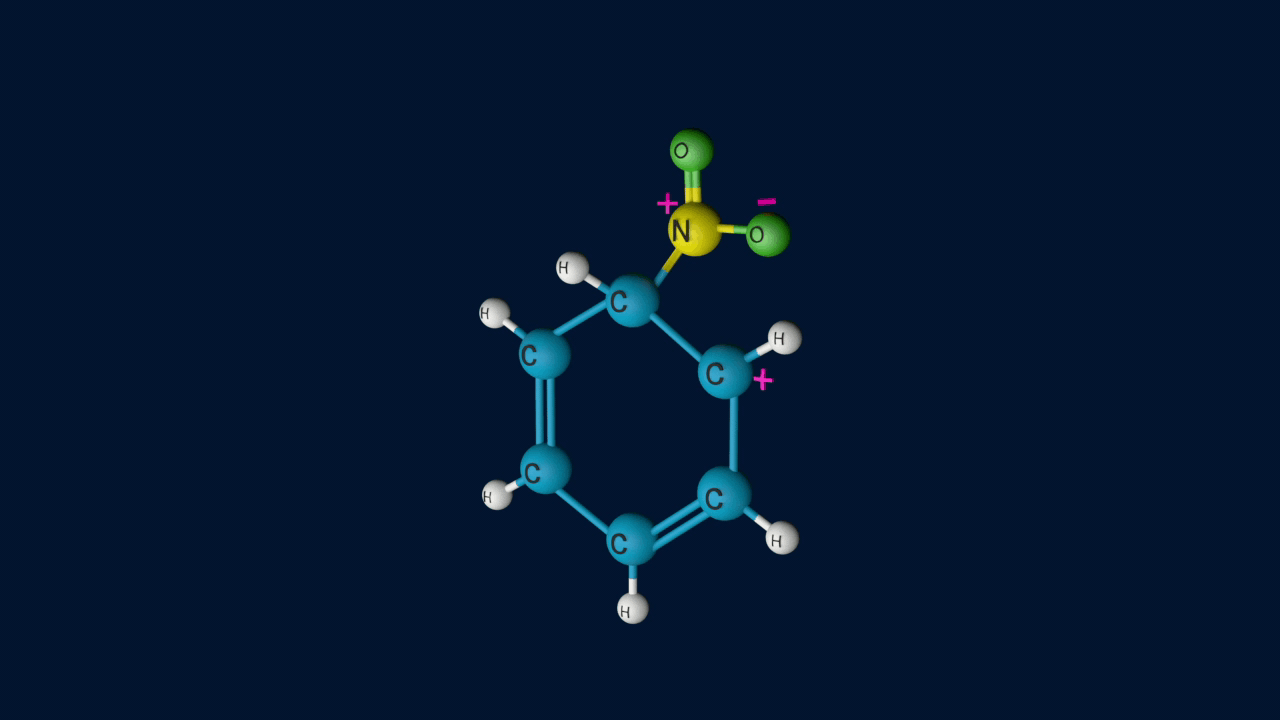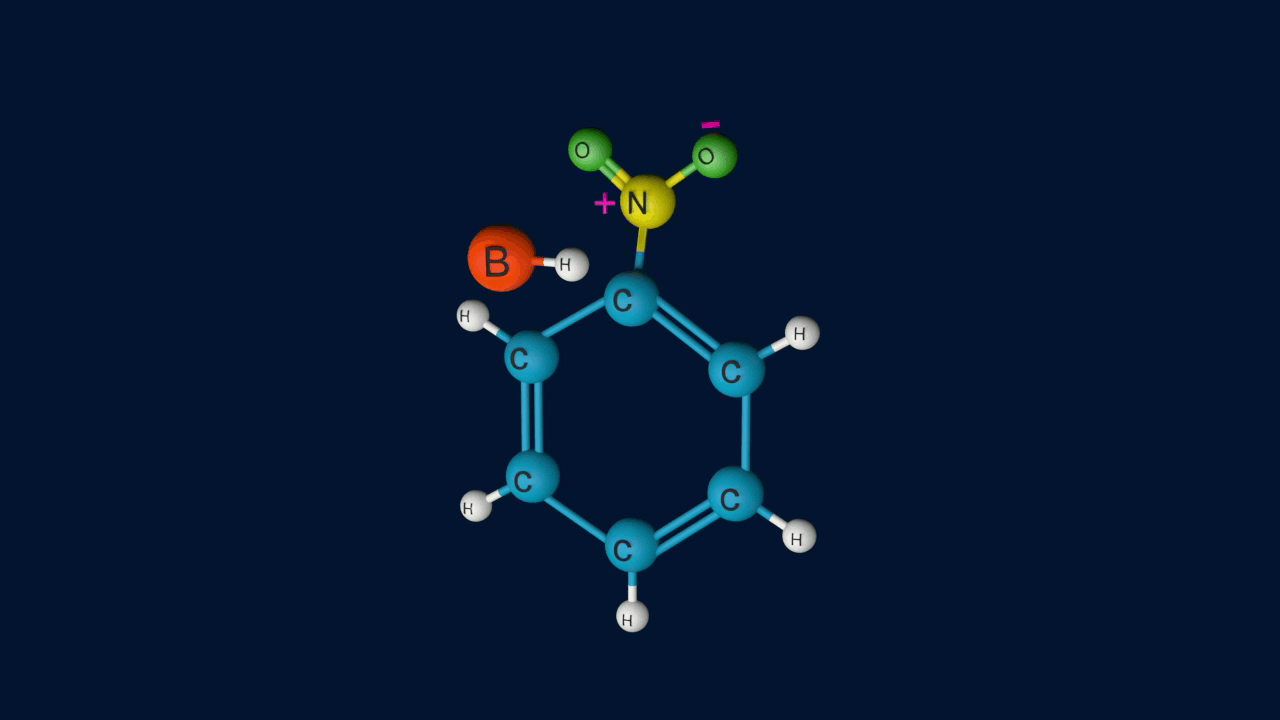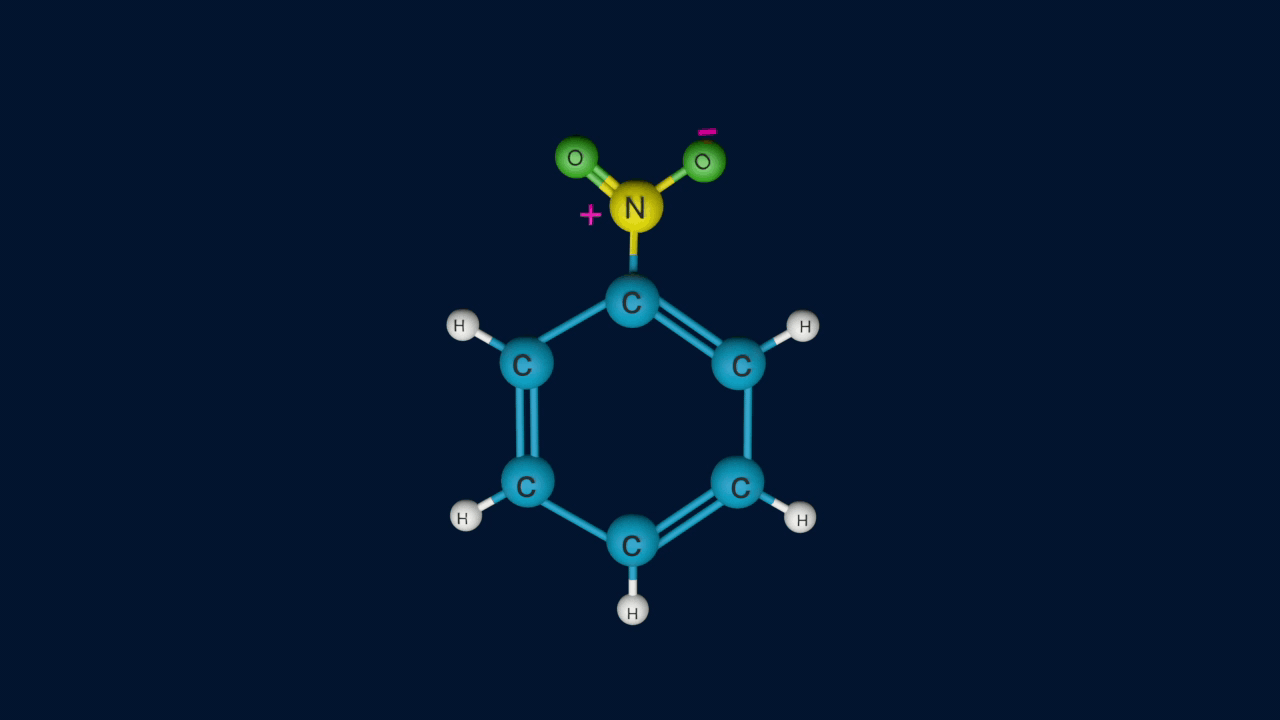
Step 2: Formation of an intermediate
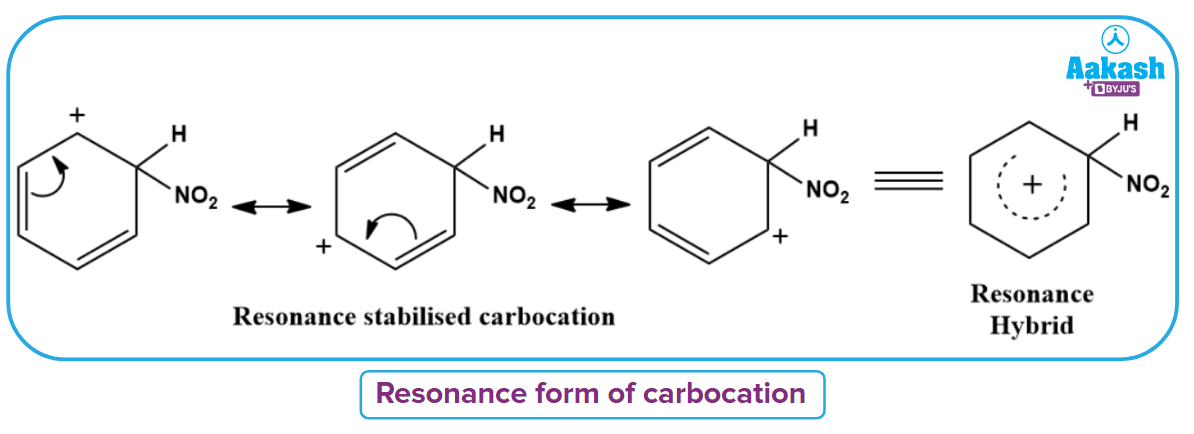
Electrophile (NO2+) is formed in the first step and attacks the benzene ring to form an intermediate carbocation which is resonance stabilised. This formation of the carbocation step is a slow and rate-determining step of nitration reaction.
The resonance forms of the formed arenium ion are as follows.
Step 3: Removal of proton from the arenium ion
In the third step, by the attack of a base, the sigma complex releases a proton from the sp3 hybridised carbon in order to restore the aromatic character, and nitrobenzene is formed as a product.
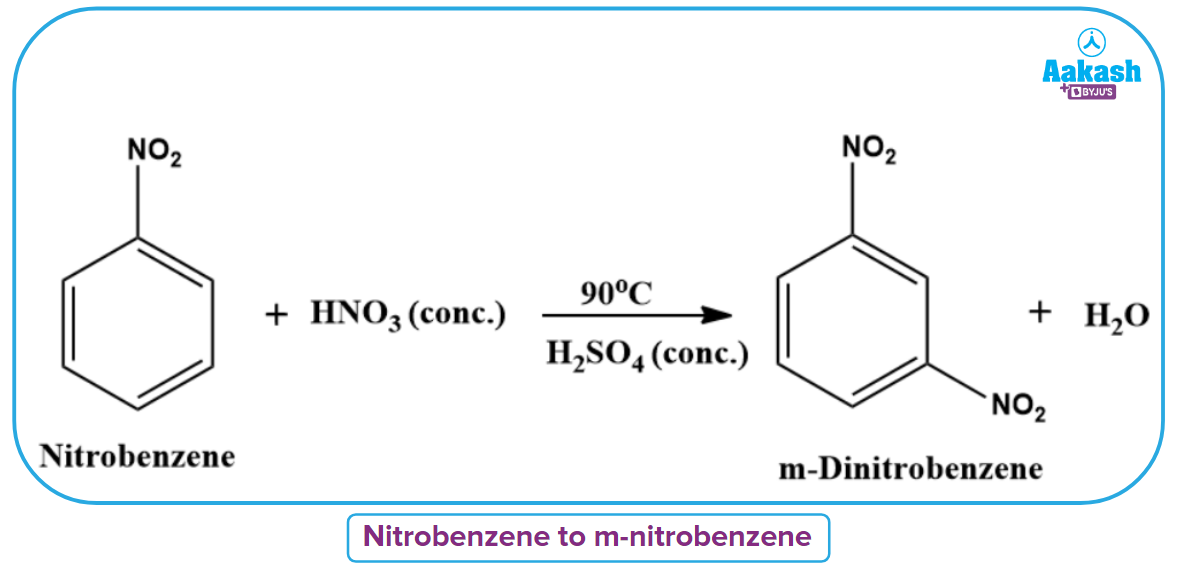
If nitrobenzene is further nitrated or benzene is treated with a nitrating mixture at 90C, m-dinitrobenzene will be formed as a product.
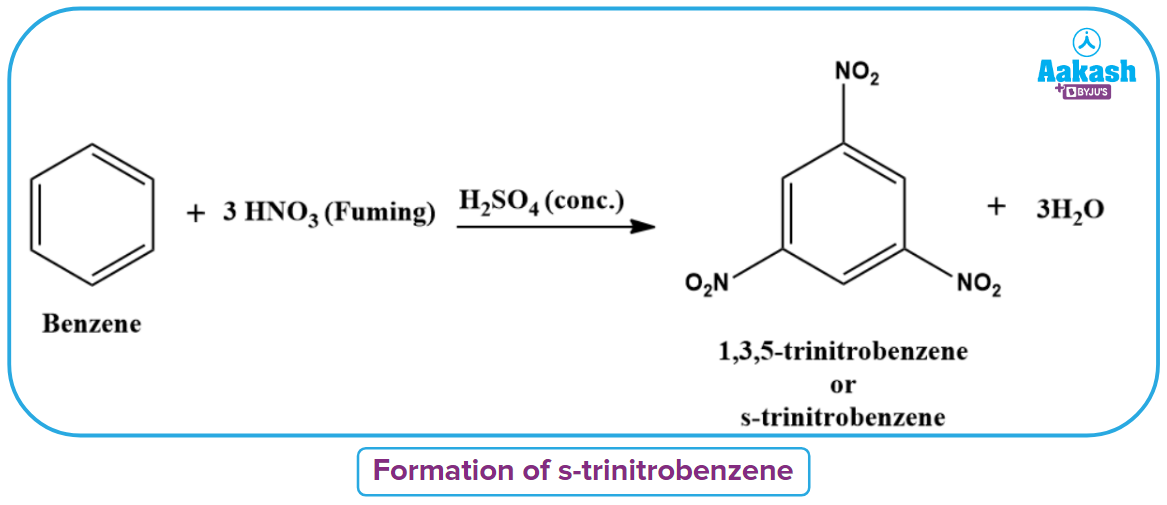
In the presence of fuming nitric acid and strong sulfuric acid, benzene can be converted into the potent explosive 1,3,5-trinitrobenzene.
Electrophilic Aromatic Sulfonation Reaction
A hydrogen atom of the benzene ring is replaced by a sulphonic acid group when benzene is treated with fuming sulfuric acid, and as a result, benzene sulphonic acid is produced. Fuming sulphuric acid is sulphuric acid that contains added sulphur trioxide (SO3). This process is called the sulphonation of benzene.
Mechanism of sulphonation
Step 1: Generation of the electrophile
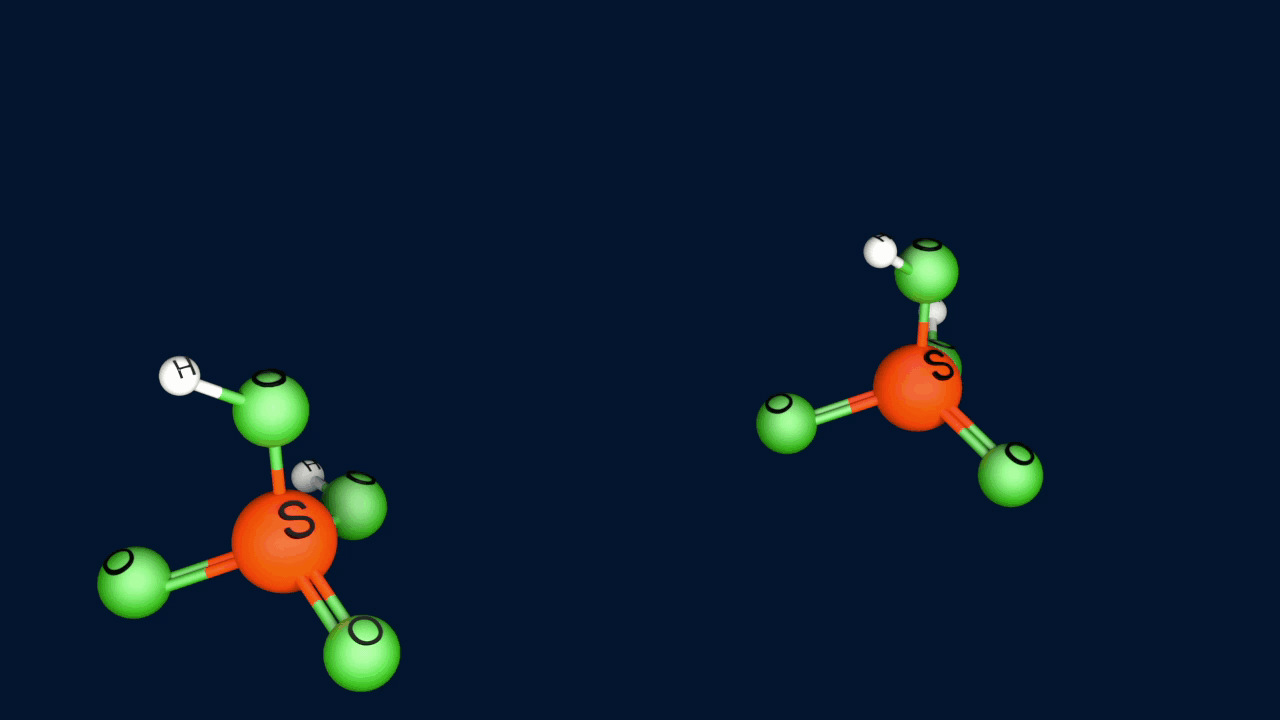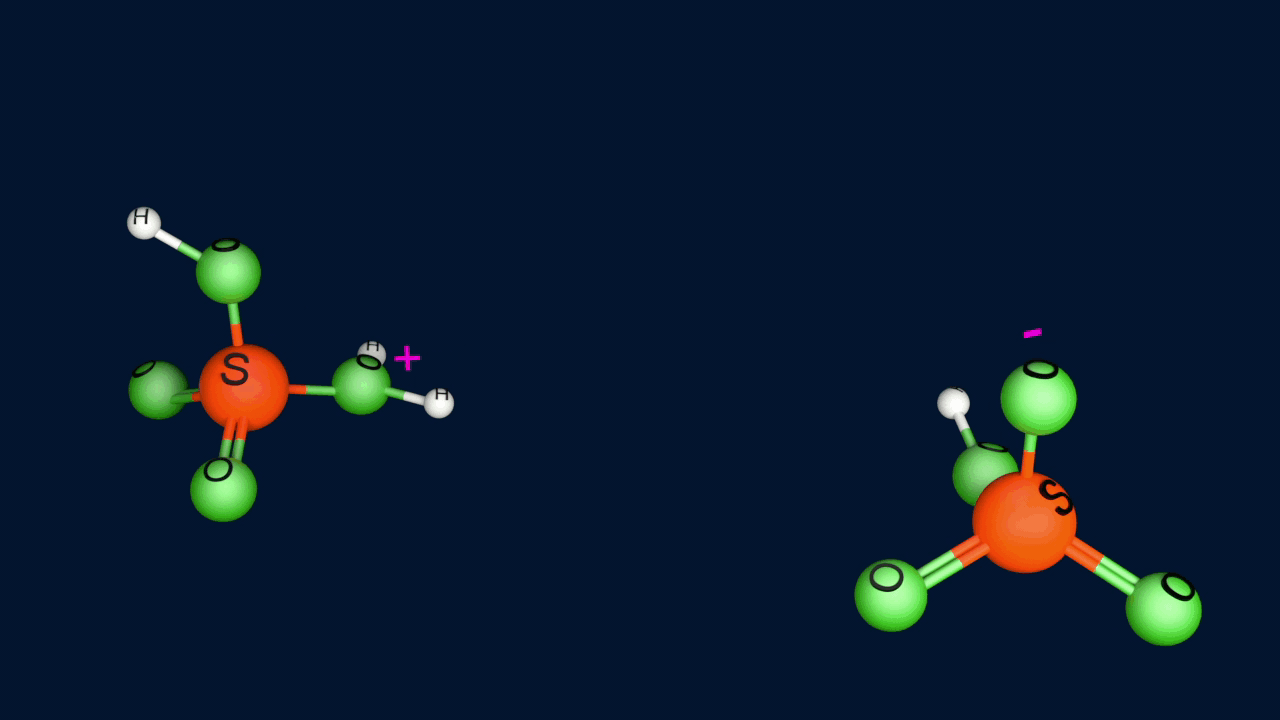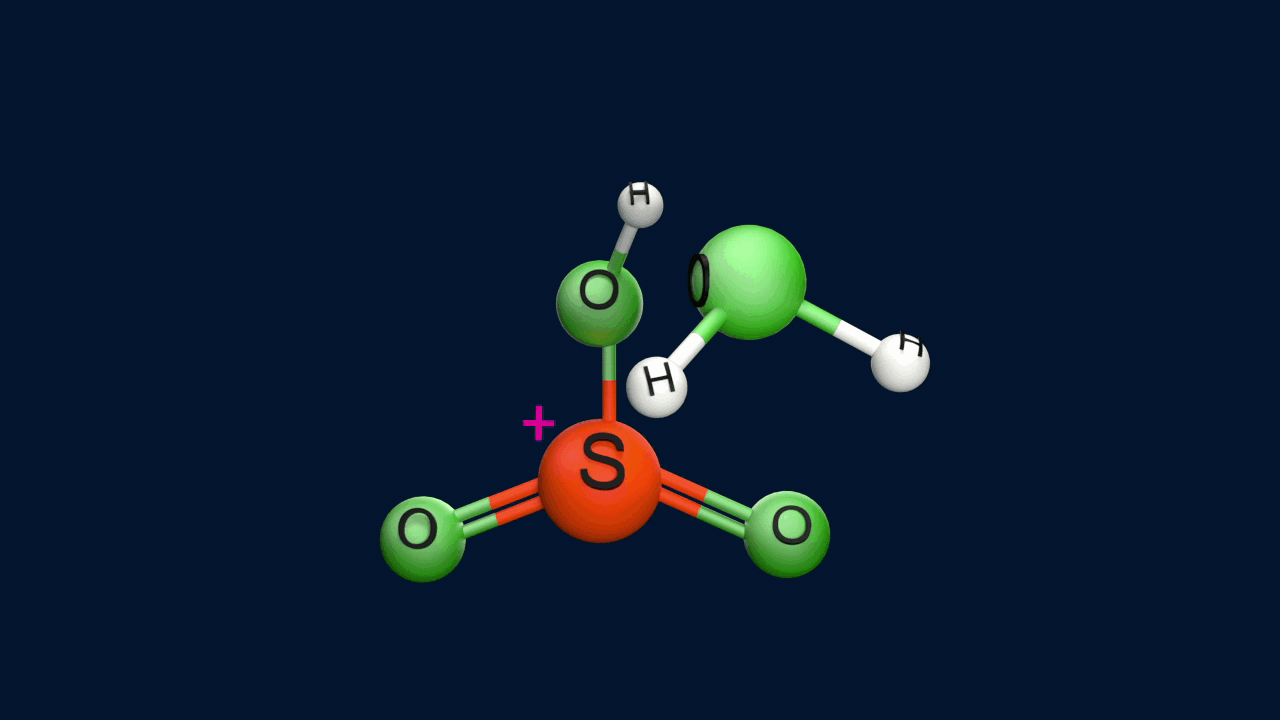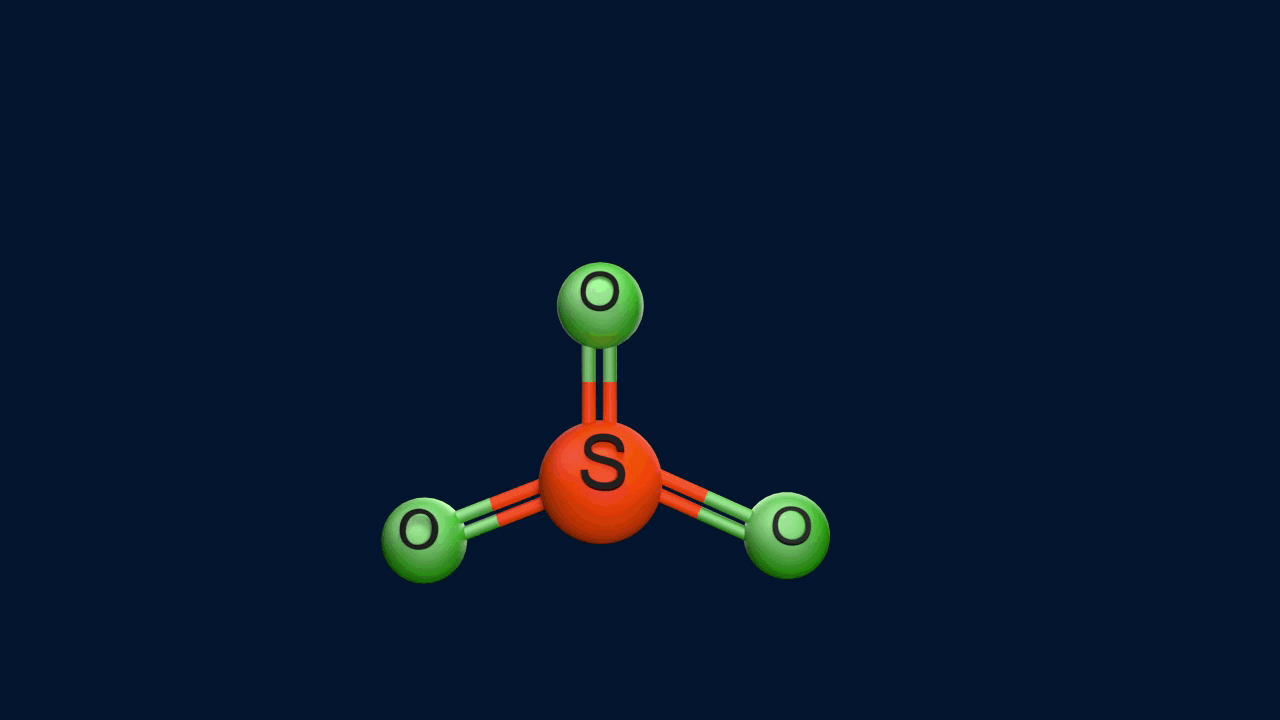
Step 2: Formation of the intermediate
Electrophile (SO3) produced in the first step attacks the benzene ring to form an intermediate carbocation, which is resonance stabilised. This formation of the carbocation step is the slow and hence rate-determining step of sulphonation reaction.
The carbocation is resonance stabilised in the following way.
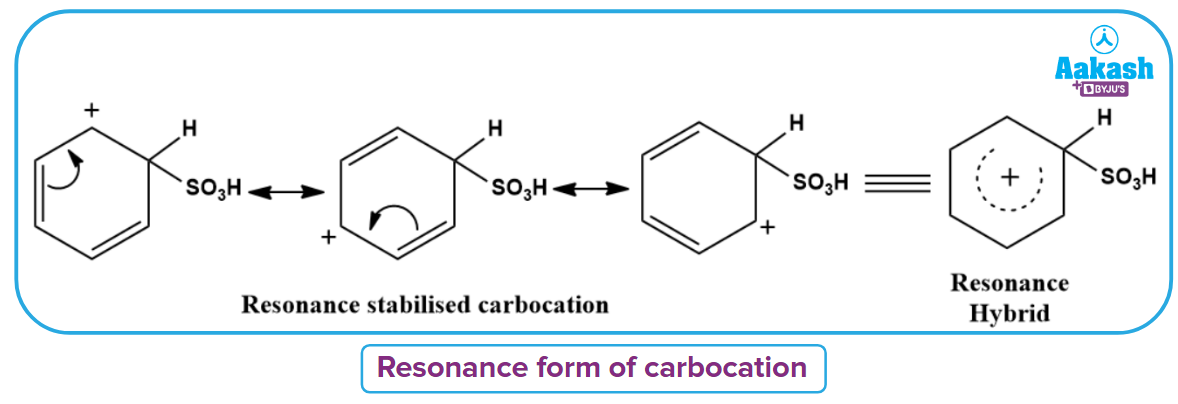
Step 3: Removal of proton from the arenium ion
In the third step, by the attack of nucleophile the sigma complex releases a proton from the sp3 hybridised carbon in order to restore the aromatic character.
Step 4: Formation of product
Lastly, the anion formed in the third step undergoes hydrolysis to yield benzene sulphonic acid as a product.
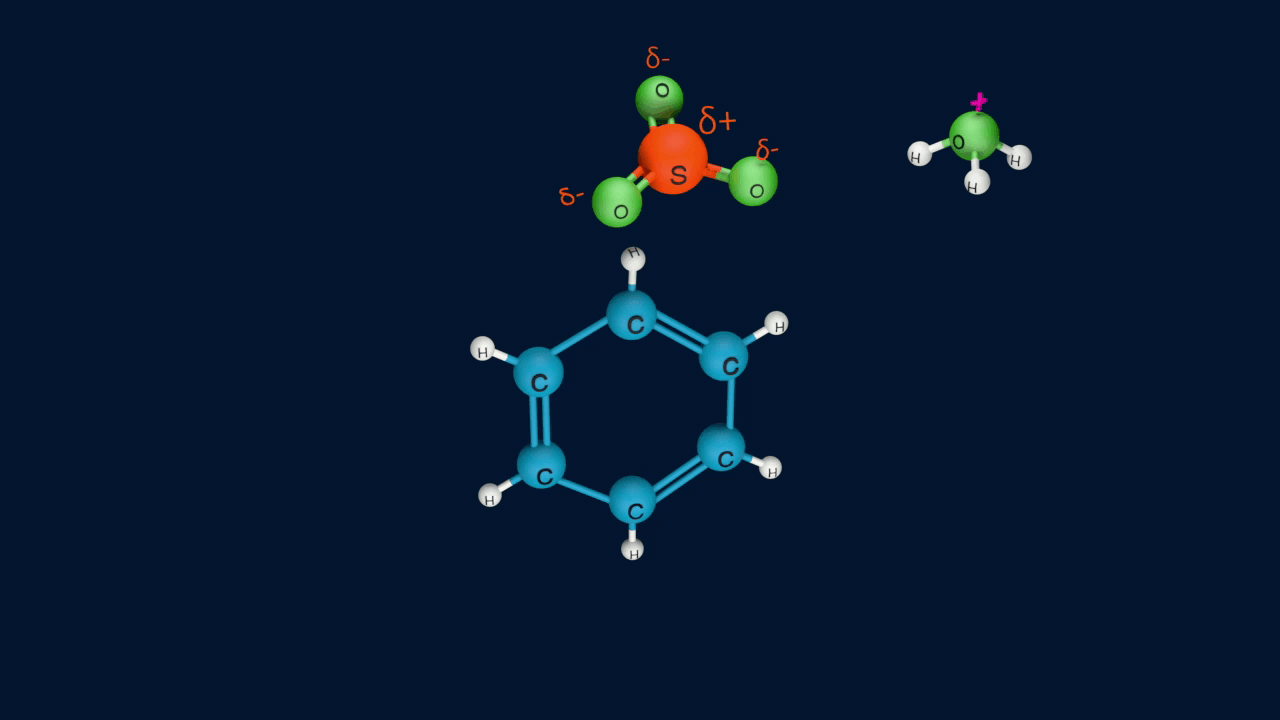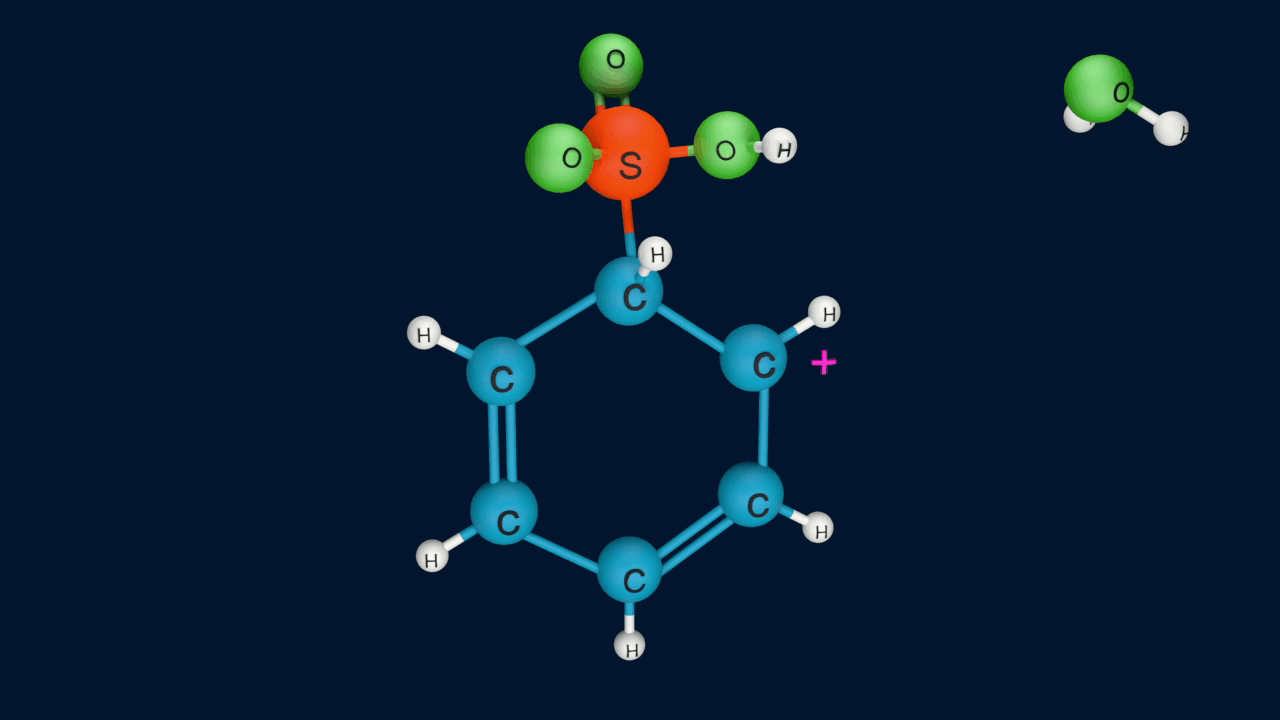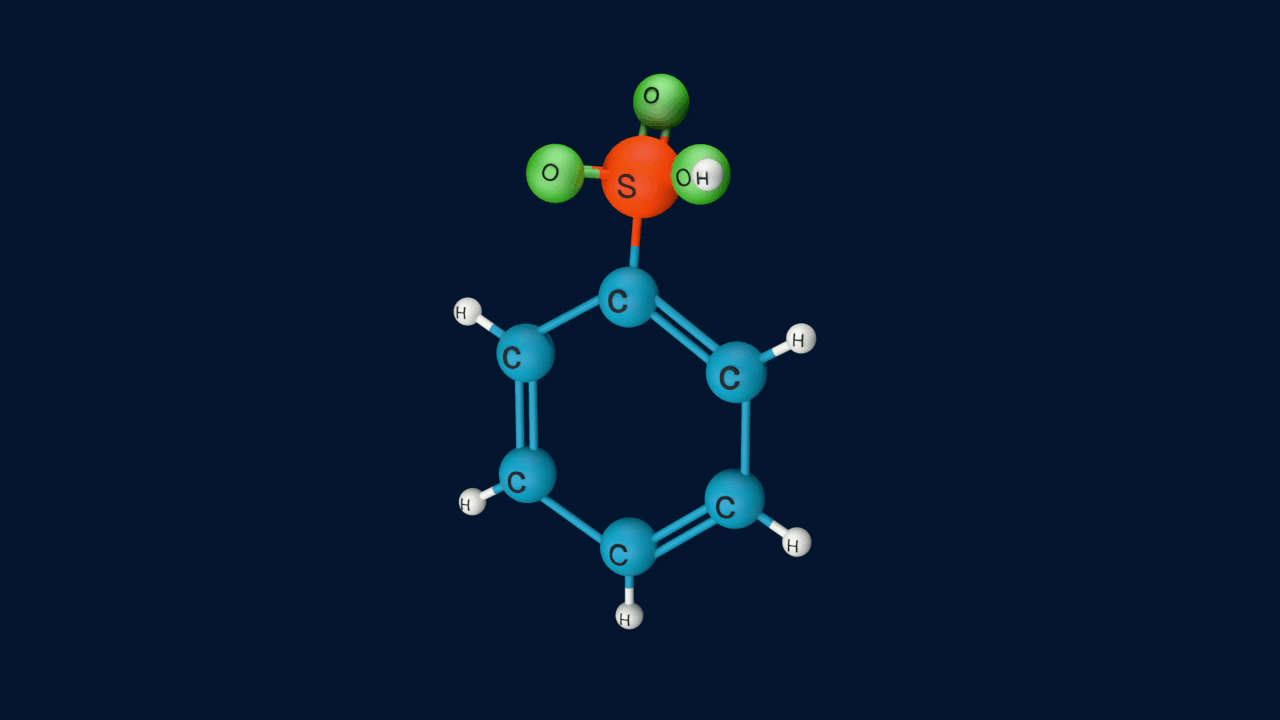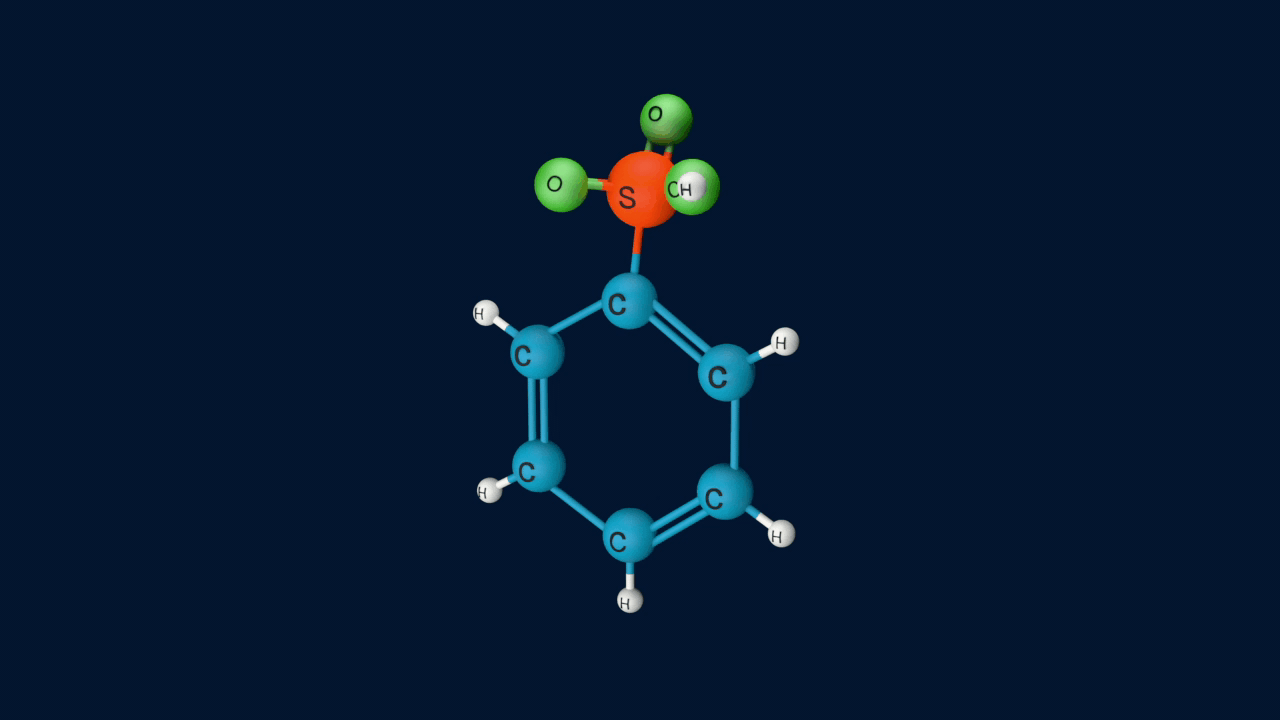
Sulphonation of benzene is a reversible reaction. If benzenesulphonic acid is heated in a dilute acid, the reaction proceeds in the reverse direction.
m-Benzenedisulfonic acid is created when benzene is heated to a high temperature and mixed with olem.
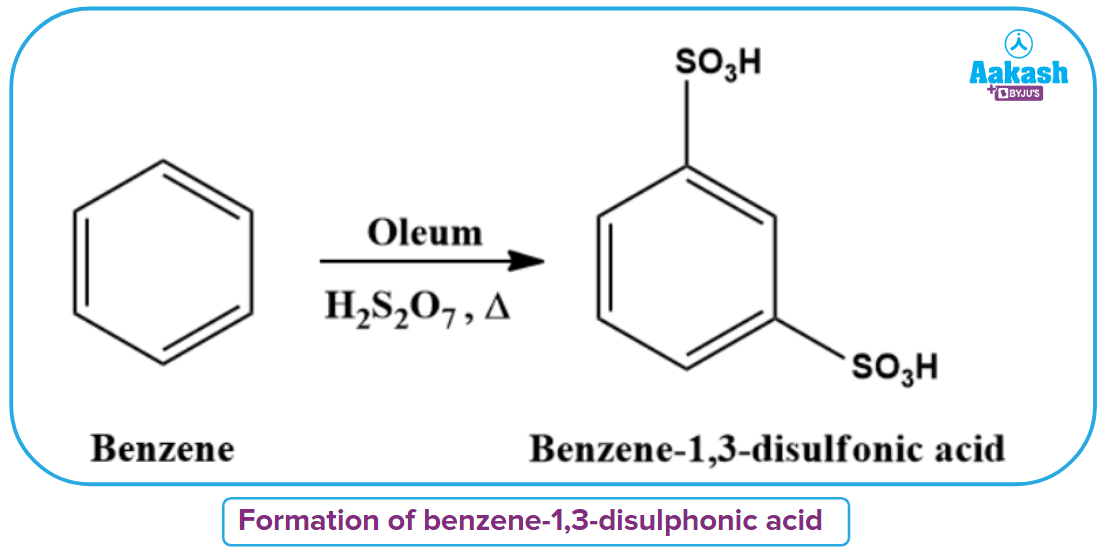
Sulphonation of benzene can also be done at room temperature using BF3 as a catalyst.
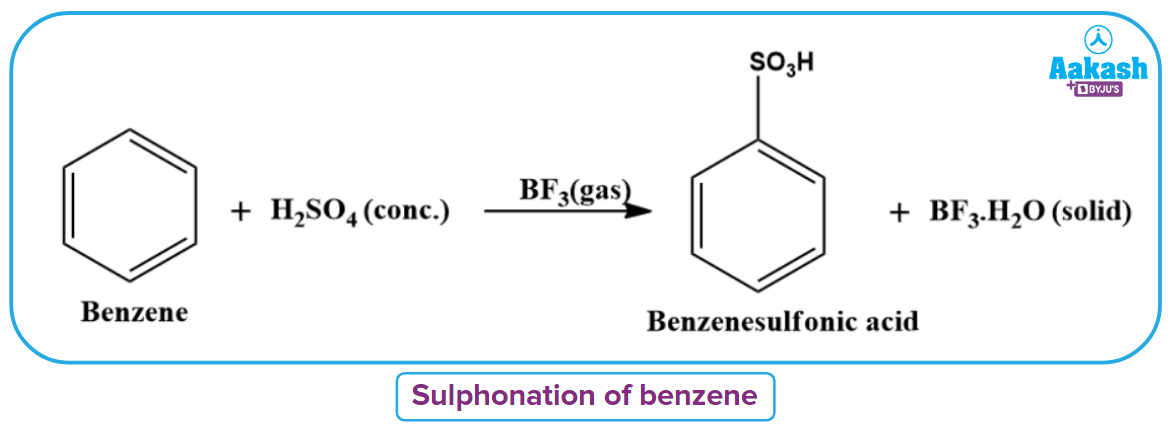
Electrophilic Aromatic Mercuration Reaction
Acetoxy mercuric benzene is produced when benzene is heated with an alcoholic solution of mercuric acetate at a high temperature, replacing a hydrogen atom of the benzene ring with the acetoxy mercuric acid group.
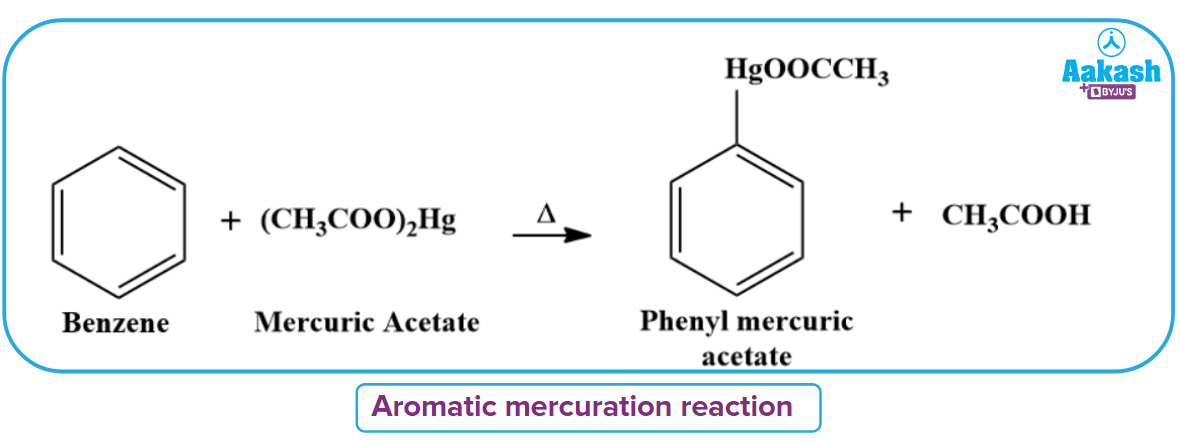
This reaction is known as mercuration and it is used in the preparation of various medicines in the pharmaceutical industry.
Formylation Reaction
The Gattermann-Koch synthesis is the formylation of benzene. The aldehyde group is immediately inserted into the benzene ring during this synthesis process. Benzaldehyde is produced as a byproduct of heating benzene in the presence of anhydrous aluminium chloride and a mixture of dry HCl gas and carbon monoxide.
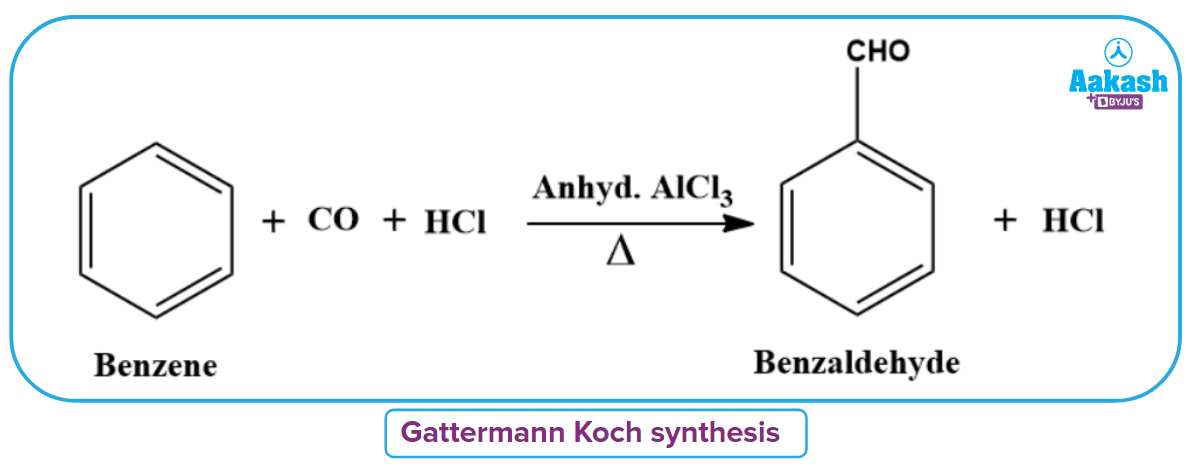
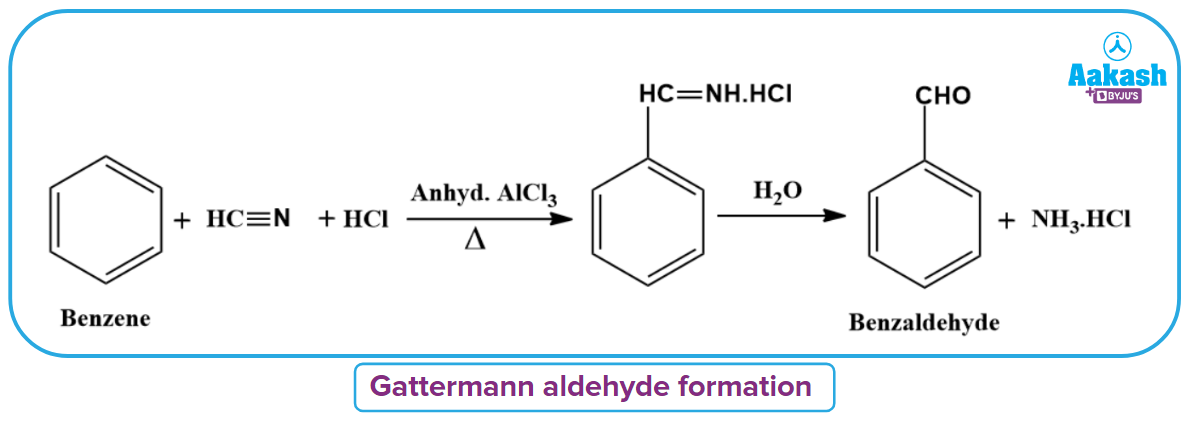
Gattermann Aldehyde Reaction
Benzene on being heated with H-CN and hydrochloric acid (HCl) in the presence of anhydrous aluminium chloride followed by hydrolysis forms benzaldehyde. H-C+=NH is utilised as an electrophile in this reaction.
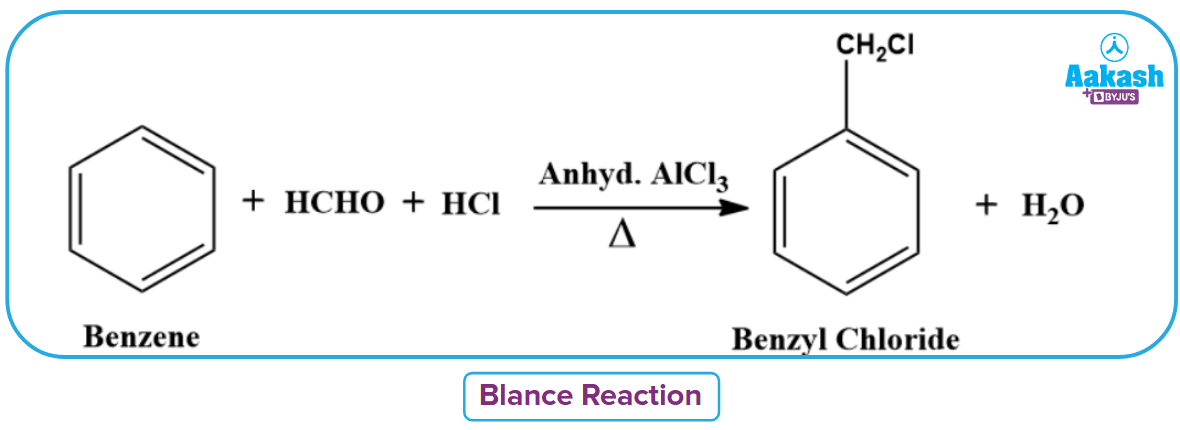
Blanc Reaction
Blanc reaction is the chloromethylation of benzene. A chloromethyl group is introduced into the benzene ring as a result of heating benzene with formaldehyde, hydrochloric acid, and anhydrous aluminium chloride, which produces benzyl chloride as a byproduct. Chloromethylation is the name of this reaction.
Recommended Videos
Nitration of benzene | CHEMISTRY | JEE | Concept of the Day | SM Sir
Friedel-Crafts Acylation | CHEMISTRY | JEE | Concept of the Day | SM Sir
Friedel-Crafts Alkylation | CHEMISTRY | JEE | Concept of the Day | Nitika Ma'am
Practice Problems
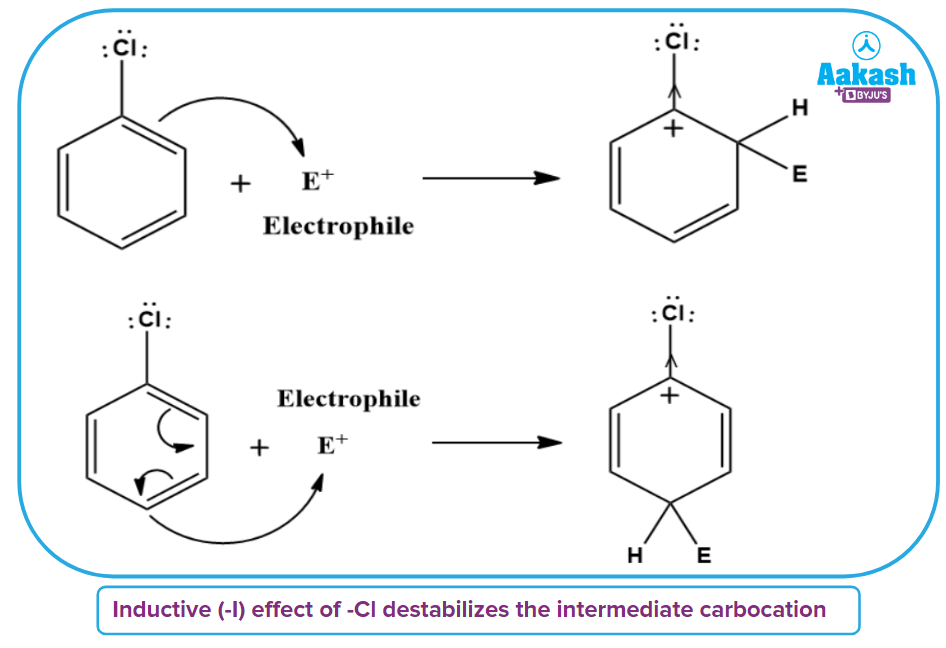
1. Although chlorine is an electron-withdrawing group, it is ortho-para directing in electrophilic aromatic substitution reactions. Why?
Solution: Chlorine withdraws electrons through the inductive effect (-I) and releases electrons through resonance (+R). Chlorine destabilises the intermediate carbocation created during the electrophilic substitution by the positive inductive effect. Chlorine tends to stabilise the carbocation by resonance, and the effect is stronger at the o- and p-positions.
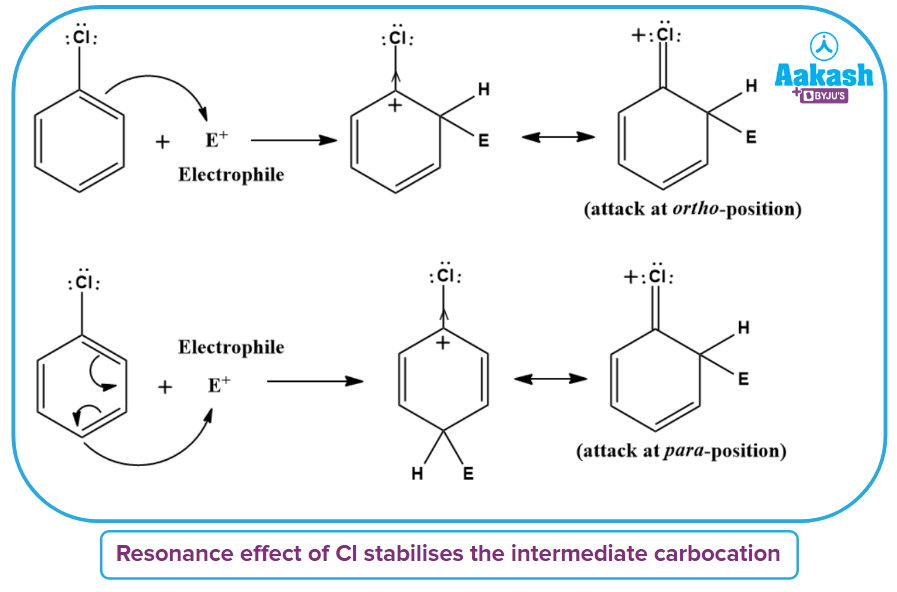
Resonance is weaker than the inductive effect, which results in net electron removal and net deactivation. Thus, the stronger inductive impact governs reactivity whereas the resonance effect governs orientation.
2. Why benzene favours electrophilic aromatic substitution reactions?
Solution: Planar molecules like benzene have electrons that are delocalized above and below the plane of the ring. It is so rich in electrons. It is therefore extremely alluring to organisms lacking in electrons, or electrophiles. It is hence particularly susceptible to electrophilic substitution reactions. Delocalized electrons are present above and below the ring's plane. The delocalized electrons give benzene its exceptional stability. Because breaking the delocalisation would result in losing the stability, benzene resists addition reactions.
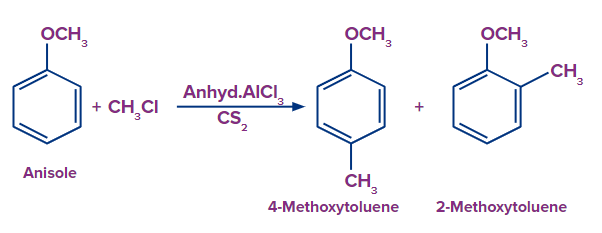
3. Anisole undergoes methylation by Friedel-Craft alkylation. The products are:
Solution: The given reaction is an electrophilic substitution reaction called a Friedel-craft alkylation. The ortho and para directing groups are the groups that direct the incoming electrophile to ortho and para positions. The density of electrons increases with ortho and para position. As a result, substitution occurs primarily on ortho and para positions. The ortho-para directing group is -OMe.
4. The compound X in the reaction is:

Answer: B
Solution: ICl with anhydrous AlCl3 will generate I+ ion which can undergo iodination easily. So, the produc formed will be iodobenzene. Chloride acts as a nucleophile. Therefore, it cannot show an electrophilic aromatic substitution reaction.
Mechanism:
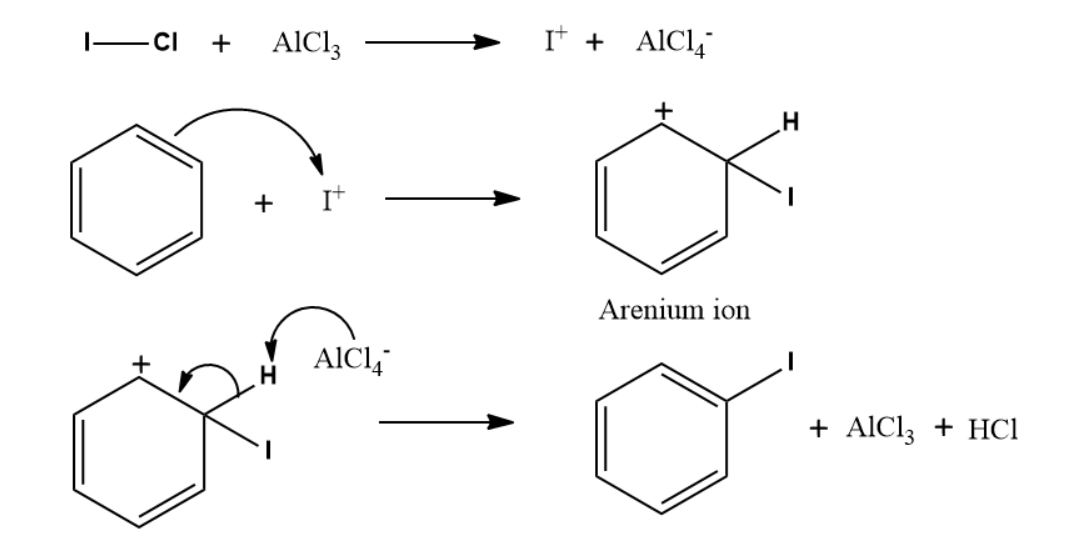
So, option B is the correct answer.
Frequently Asked Questions – FAQ
1. What is the basic difference between electrophilic and nucleophilic substitution reactions?
Answer: In a substitution reaction, an atom or group of atoms in an organic molecule are directly swapped out for another atom or set of atoms, leaving the rest of the molecule unaffected. Electrophiles are the ones who start electrophilic substitution processes. Reactions involving substitution that start with a nucleophilic attack are known as nucleophilic substitution reactions.
2. What is the alternate name of the arenium complex?
Answer: The second phase of the electrophilic aromatic substitution process produces an intermediate carbocation known as an arenium ion, also known as a wheland complex or a Meisenheimer complex.
3. What are some of the examples of aromatic electrophilic substitution reaction?
Answer: There are numerous different types of electrophilic aromatic substitution, but some of the more prevalent ones include halogenation, nitration, and sulphonation of benzene.
4. How is aromatic electrophilic substitution reactions useful?
Answer: The electrophilic aromatic substitution reaction is among the most significant in synthetic organic chemistry. Important intermediates that can be employed as precursors in the development of medicinal, agrochemical, and industrial products are created by these processes.