-
Call Now
1800-102-2727
Reactions of Alkynes - Acidic Nature, Hydrogenation, Halogenation, Addition of water, Combustion, Ozonolysis, Hydroboration Oxidation, Practice Problems and FAQs
Reactions of Alkynes - Acidic nature, Hydrogenation, Halogenation, Addition of water, Combustion, Ozonolysis, Hydroboration Oxidation, Practice Problems and FAQs
Have you ever heard of the gas acetylene. Acetylene is widely used in welding and cutting, which is an important process in different manufacturing industries. The welding process using acetylene is known as oxy-acetylene welding. This method is used for cutting or welding materials which require temperatures of up to 35000C. Acetylene is capable of producing the hottest flame of all other gasses.
The IUPAC name of this gas is ethyne, which means it belongs to a class of hydrocarbons known as alkynes, about the reaction of alkynes we are going to discuss further.
Table of Contents
- Structure of Alkyne
- Acidic nature of alkynes
- Catalytic Hydrogenation of Alkyne
- Hydrogenation of Alkynes
- Halogenation to alkyne
- Addition of water to alkynes
- Addition of water in internal unsymmetrical alkynes
- Combustion of Alkynes
- Ozonolysis of alkynes
- Hydroboration Oxidation reaction
- Practice Problems
- Frequently Asked Questions
Structure of Alkyne
The Alkyne molecule is sp hybridized with two pi bonds. Due to the presence of two weak pi (π) bonds, alkynes undergo addition reaction. Alkynes add up to two molecules of hydrogen, halogens, hydrogen halides, etc.
General Formula of Alkyne is
An illustration of orbital overlap in alkynes representing the weak pi (π) electron cloud is given in
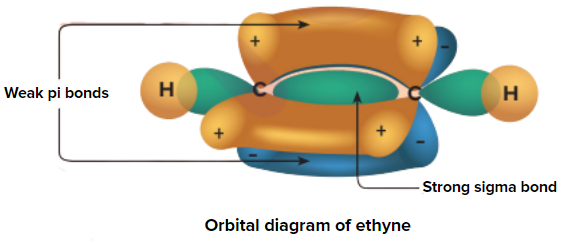
Acidic nature of alkynes
Sodium metal (Na) and sodamide (NaNH2; strong base) react with terminal alkynes like ethyne to form sodium acetylide () with the liberation of dihydrogen (H2) and ammonia gas (NH3), respectively.
Example 1:
Ethyne Monosodium ethynide
Disodium ethynide
Example 2:
Propyne Sodium propynide
Ethyne Monosodium ethynide
These reactions are not observed in the case of ethane (alkane) or ethene (alkene), indicating
that ethyne (terminal alkyne) is acidic in nature when compared to ethane and ethene.
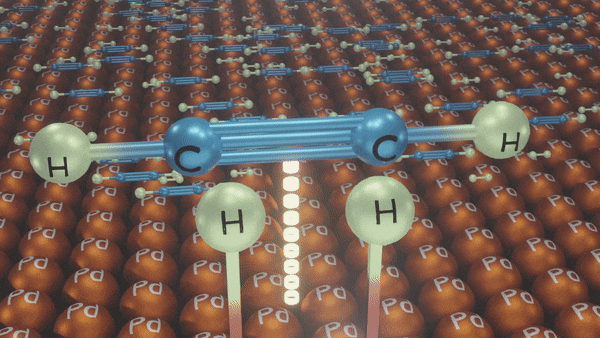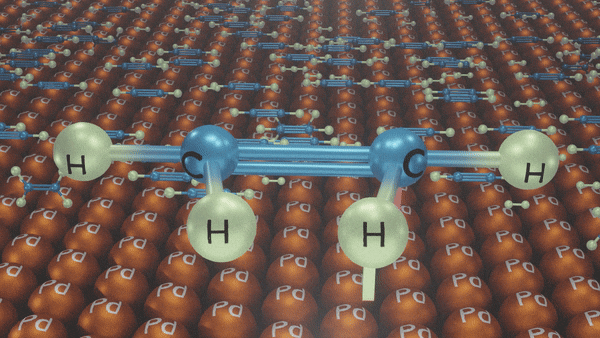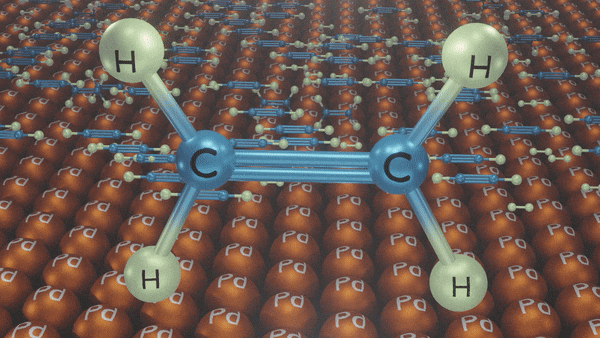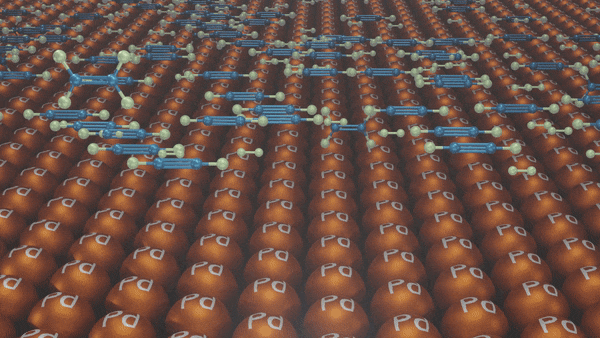
Catalytic Hydrogenation of Alkyne
An addition of dihydrogen to alkynes in the presence of Ni/Pd/Pt gives alkanes.
For example,
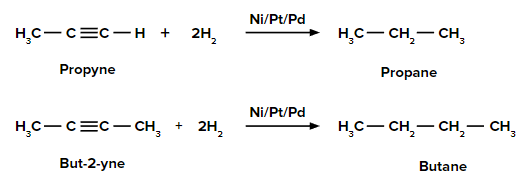
Partial hydrogenation (reduction) of alkynes
- While adding H2 to alkynes, if the reduction is to be stopped at the alkene stage, then
the catalyst needs to be poisoned.
- Palladium catalyst () deliberately poisoned with lead or sulphur compounds or quinoline, etc., is known as Lindlar’s catalyst.
Reduction of propyne to propane and propene
In the presence of a palladium catalyst, propyne is reduced to propane. When the reduction is carried out with palladium poisoned with quinoline, propyne is reduced to propene.
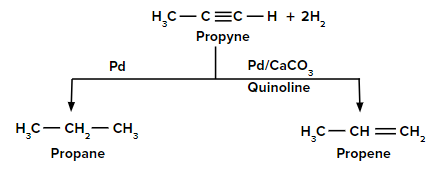
Hydrogenation of Alkynes
- Lindlar’s Catalysts
Reagents: , quinoline
Poisoned palladium catalyst: It is composed of powdered calcium carbonate coated with palladium and poisoned with quinoline to reduce its catalytic activity so that a complete reduction of alkynes does not take place. Lindlar’s catalyst is used to carry out partial reduction of alkynes to alkenes. Poisoning deactivates the catalytic activity to an extent and the reduction of the alkyne is restricted to the formation of an alkene.

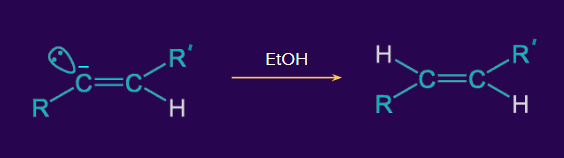
- Birch reduction
The conversion of alkyne to alkene using Na/liquid NH3 in the presence of EtOH is known as the Birch reduction. This is a trans addition and trans alkene is formed in the birch reduction.
Example:
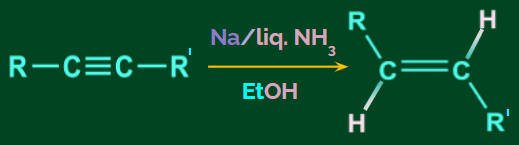
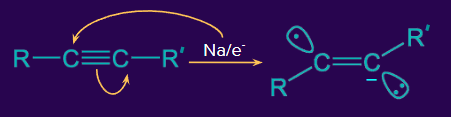
Mechanism:
Step 1: A π-electron shift takes place in non-terminal alkynes in the presence of the electron of sodium metal.
Step 2: The alkenyl radical anion accepts H+ from EtOH.
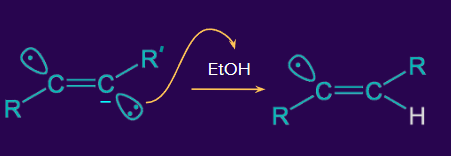
Step 3: The alkenyl radical reacts again with the electron from the sodium metal.
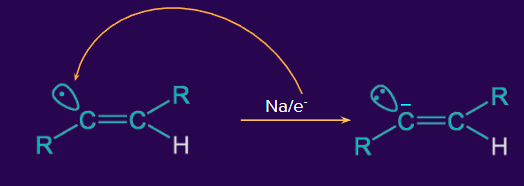
Step 4: The alkenyl anion accepts H+ from EtOH and is finally converted into a trans alkene.
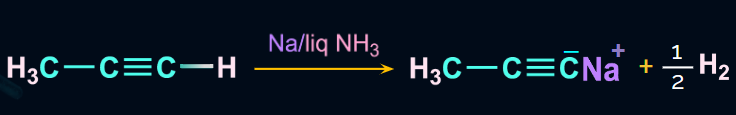
Terminal alkynes do not show the Birch reduction. Propyne cannot give propene on reacting with
Na/liquid NH3. Instead, they show a redox reaction due to the acidic nature of the terminal hydrogen. Propyne reacts with Na/liquid NH3 and gives sodium propynide and hydrogen gas. This reaction is a redox reaction.
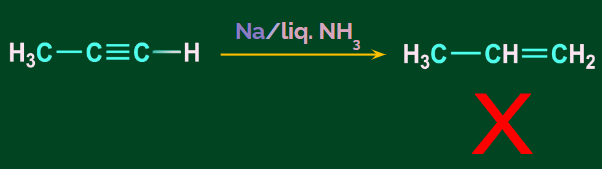
Halogenation to alkyne
- Alkynes react with a molecule of X2 (in ) to form 1,2-dihaloalkenes.
- Further, the reaction of one more molecule of X2 to 1,2-dihaloalkene produces tetra halogenated products.
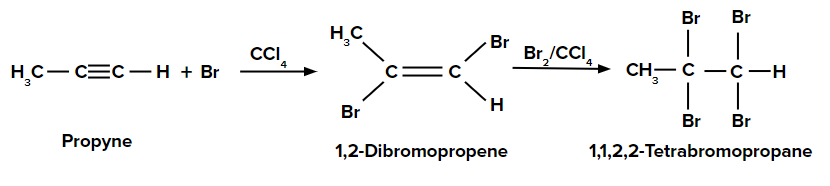
Addition of Br2 (in CCl4) to propyne
When the first molecule of Br2 in CCl4 is added to propyne, an anti-addition reaction happens since the two substituents are added on the opposite sides of the pi bond.
Test for unsaturation with Br2 in CCl4
The reddish-brown color of bromine solution in carbon tetrachloride is discharged when
bromine adds up to an unsaturated site.
- Hence, this reaction is used as a test for unsaturation.
- Alkenes and alkynes decolourise the reddish-brown color of Br2 / CCl4
We have hexane (C6H14), hexene (C6H12), and hexyne (C6H10) in three test tubes. When Br2 / CCl4 is added to hexane, the reddish brown color of Br2 / CCl4 is retained. When Br2 / CCl4 is added to hexene, the reddish-brown color is discharged (decolourised) and 1,2-dibromohexane is formed. Similarly, when Br2 / CCl4 is added to hexyne, the reddish-brown color is discharged (decolourised) and 1,1,2,2-tetrabromohexane is formed.

Test for unsaturation with Br2 / CCl4
Electrophilic Addition to alkyne:
Let us consider the addition of any compound (HZ) to an alkyne.
Step 1: Addition of electrophile
When we add one equivalent of an electrophile (let’s say H+) to an alkyne, it forms a vinyl carbocation. This is an electrophilic addition reaction. When a protic acid (HX) is added to an asymmetric alkene, the π electrons shift in such a way that the positive charge is present on the carbon which is attached to the alkyl group so that the carbocation is stabilized by the +I effect of the alkyl group and also by the hyperconjugation of respective ⍺-hydrogen atoms.
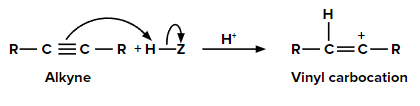
Step 2: Addition of nucleophile (Z-) to vinyl carbocation
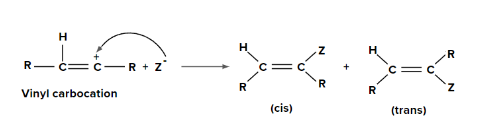
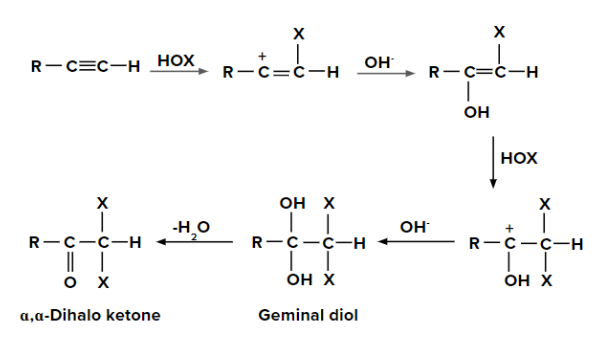
(i) Addition of hypohalous acid to alkynes
- Since the electronegativity of oxygen is higher than that of halogen except for fluorine, HOX breaks into OH- and X+.
- Two molecules of HOX (HOCl, HOBr or HOI) get added to alkynes and form geminal diols.
- This geminal diols form ⍺,⍺-dihalo ketones (two halogen groups are present at ⍺-position with respect to carbonyl group) on the elimination of H2O.
- The geminal diols are unstable. Therefore, they are easily converted to their respective ketones or aldehydes by dehydration (loss of one water molecule).
Generally, the -OH group is added to that carbon atom of alkyne, where more alkyl groups are present.
When HOX is added to the given alkyne, the pi electrons shift in such a way that the positive charge
is present on the carbon which is attached to the alkyl (-R) group so that the positive charge is stabilized
by +I effect of the alkyl group. When the second molecule of HOX is added to the alkene formed
previously, the pi electrons shift in such a way that a positive charge is present on the carbon which is
attached to the -OH group as the positive charge is stabilized by the +M effect of the -OH group.
(ii) Addition of hydrogen halides to alkynes
When two molecules of HX (HCl, HBr, or HI) are added to the alkynes, geminal dihalides (in which two halogen atoms are attached to the same carbon atom) is formed.
According to Markovnikov's rule, the addition of HX to unsymmetrical alkynes takes place.
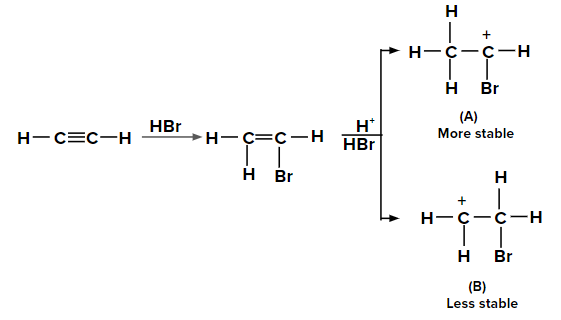
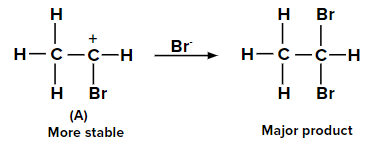
When the second molecule of HBr is added to bromoethene, two possible carbocations can be
formed. The carbocation labeled as (A) is more stable than (B) due to the +M effect of the -Br
group and also by the hyperconjugation of three ⍺-hydrogen atoms. Hence, the major product is
obtained from carbocation (A).
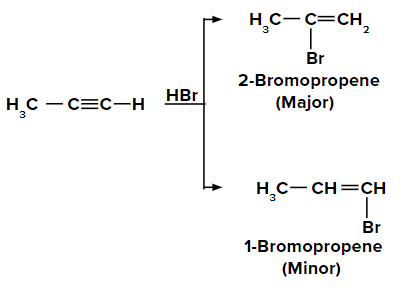
Adding HBr to propyne according to the Markovnikov rule
On adding the first molecule of HBr to propyne, we obtain 2-bromopropene and
1-bromopropene as the major and minor products respectively, due to the Markovnikov rule.
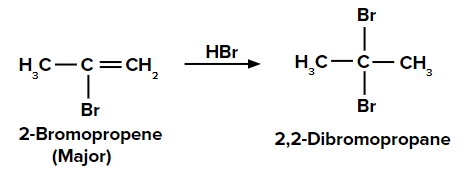
When the second molecule of HBr is added to 2-bromopropene, we get the final product as
2,2-dibromopropane.
(iii) Addition of water to alkynes
Like alkanes and alkenes, alkynes are also immiscible in water. However, one molecule of water adds to an alkyne on heating with HgSO4 and diluted H2SO4 at 333 K to form a carbonyl compound.
This addition of water to alkynes is named as Kucherov Reaction.
The addition of water to alkynes happens according to the Markovnikov rule.
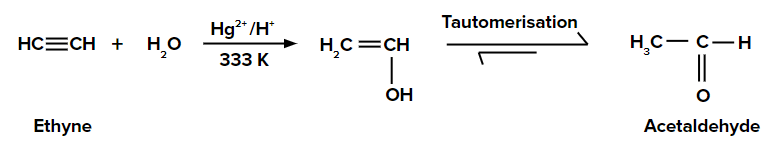
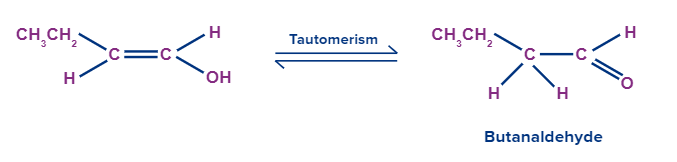
Adding H2O to ethyne
An addition of H2O to ethyne gives an enol compound. This enol compound tautomerizes to the aldehyde (i.e., acetaldehyde is formed).
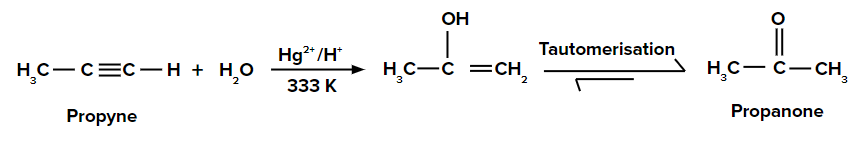
Adding H2O to propyne
When H2O is added to propyne, the π electrons in propyne shift in such a way that the positive charge is present on that carbon which is attached to the -CH3 group so that the carbocation formed is stabilized by the +I effect of the -CH3 group and also by the hyperconjugation of three ⍺-hydrogen atoms. Thus, H2O attacks the carbocation. We obtain an enol compound that tautomerizes to the keto compound (propanone).
Addition of water in internal unsymmetrical alkynes
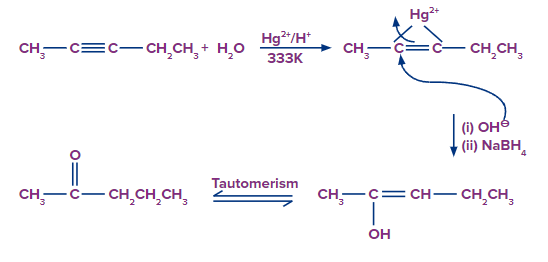
Adding H2O to Pent-2-yne
- When H2O is added to pent-2-yne, the π electrons shift in such a way that the positive charge is present on that carbon which is attached to the alkyl group so that the carbocation formed is stabilized by the +I effect of the alkyl group and also by the hyperconjugation of respective three ⍺-hydrogen atoms. Thus, H2O attacks the carbocation. We obtain an enol compound that tautomerizes to the keto compound.
Combustion of Alkynes
Complete Combustion Reaction
Complete combustion reactions, also known as clean combustion reactions, entail the complete oxidation of the fuel (usually a hydrocarbon). Such reactions frequently produce only water and carbon dioxide as byproducts. The combustion of wax candles is a common example of a clean combustion reaction.
Incomplete Combustion Reaction
Incomplete combustion reactions (also known as 'dirty' combustion reactions) are combustion reactions that produce byproducts such as ash and soot. It is common for such combustion reactions to produce carbon monoxide (a highly poisonous gas with the chemical formula CO) and water.
Complete Combustion of Alkynes
The general reaction for the complete combustion of alkynes is given as follows:
|
Alkyne |
Complete combustion reaction |
Balanced Complete combustion reaction |
|
C2H2 |
||
|
C3H4 |
||
|
C4H6 |
||
|
C5H8 |
Incomplete Combustion of Alkynes
The general reaction for the incomplete combustion of alkynes is given as follows:
|
Alkyne |
Incomplete combustion reaction |
Balanced Incomplete combustion reaction |
|
C2H2 |
||
|
C3H4 |
||
|
C4H6 |
||
|
C5H8 |
Ozonolysis of alkynes
Alkynes contain 2 pi bonds. The triple bond of alkynes, in the presence of an ozone molecule, undergoes oxidative cleavage. Alkynes undergo oxidation resulting in the formation of end products such as diketones and acid anhydrides. In the presence of water, the acid anhydride gives rise to two carboxylic acids with the help of hydrolysis.
Mechanism of ozonolysis reaction of alkyne
The reaction mechanism of ozonolysis involves 3 steps.
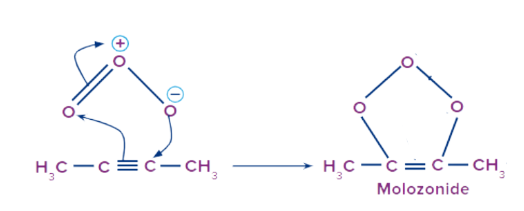
Step 1: Attack of the ozone molecule
The ozone molecule consists of 3 oxygen atoms. One of the oxygen atoms acquires a partial negative charge and the other one acquires a partial positive charge. The negatively charged oxygen ion attacks the triple-bonded carbon atom. The ozone molecule gets attached to the reactant. Rearrangement occurs within the structure resulting in the formation of an intermediate structure called the molozonide.
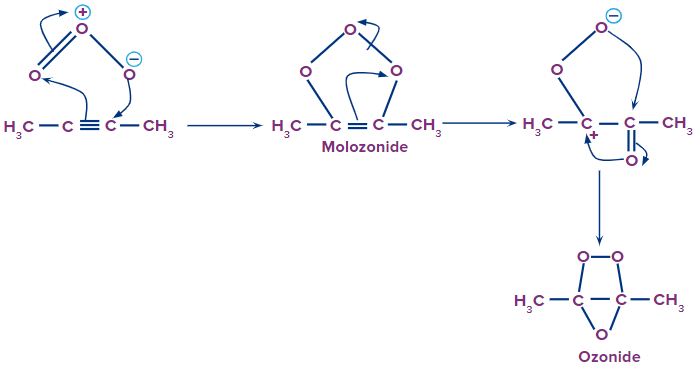
Step 2: Formation of ozonide intermediate
The molozonide intermediate further rearranges itself resulting in the formation of a stable ozonide intermediate. In ozonide, we can see that the triple bond is reduced to a single bond. Later on, the single bond between two carbon atoms also breaks, yielding different carbonyl compounds.
Formation of Ozonide
Step 3: Formation of carbonyl compounds
It can be performed by two types of mechanisms, which are given as follows:
- Reductive ozonolysis
- Oxidative ozonolysis
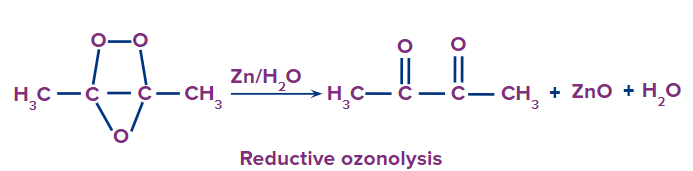
Reductive ozonolysis
This type of ozonolysis of alkyne involves the addition of an ozone molecule to an alkyne to form an ozonide. Ozonide is unstable and decomposes easily by reduction into , -diketones. The reagent that is used in reductive ozonolysis is given as follows:
(i) Zn and H2O
(ii) (CH3)2S and H2O
The formation of , can be given as:
Oxidative Ozonolysis
This type of ozonolysis of alkynes involves the addition of an ozone molecule to an alkyne to
form an ozonide, followed by the oxidation of the ozonide to smaller oxidized molecules like acid. The reagent which is used in oxidative ozonolysis is given as follows:
(i) H2O
(ii) H2O2
The formation of carboxylic acids can be given as:
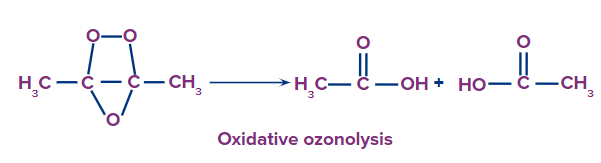
In oxidative ozonolysis, if formic acid will form, it will further decompose into carbon dioxide and water
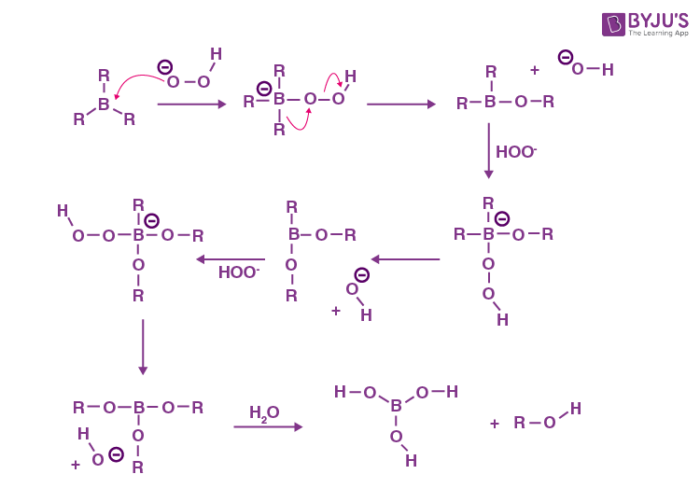
Hydroboration Oxidation reaction
The hydroboration oxidation reaction is an organic reaction that is used to convert alkenes into neutral alcohols or alkynes into ketones or aldehydes. This is accomplished through a two-step procedure that includes a hydroboration step and an oxidation step. This is accomplished through a net addition of water (across the entire double bond).
Mechanism
The mechanism of hydroboration oxidation can be thought of as an anti-Markovnikov reaction in which a hydroxyl group attaches itself to the less substituted carbon. Herbert Charles Brown, an English-born American chemist, reported the hydroboration oxidation reaction for the first time in the second half of the 1950s.
For this work, he was awarded the Nobel Prize in Chemistry in 1979.
The conversion of alkynes into carbonyl compounds takes place here. The entire reaction can be broken down into two steps, as explained below.
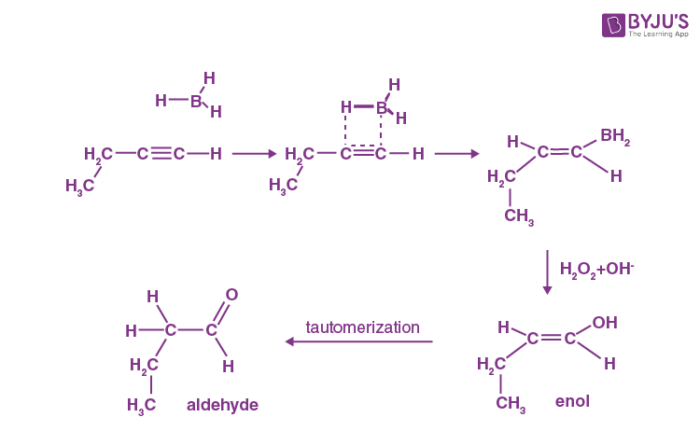
Step 1: Hydroboration
In an anti-Markovnikov way, the terminal alkynes can undergo hydroboration. The less substituted carbon, which is also the least hindered, becomes a priority target for the boron atom's attack. A bulky reagent of borane must be used to stop the reaction at the alkenyl group attached to the borane stage. If borane is used alone, it will result in the hydroboration of both the alkyne's pi bonds.
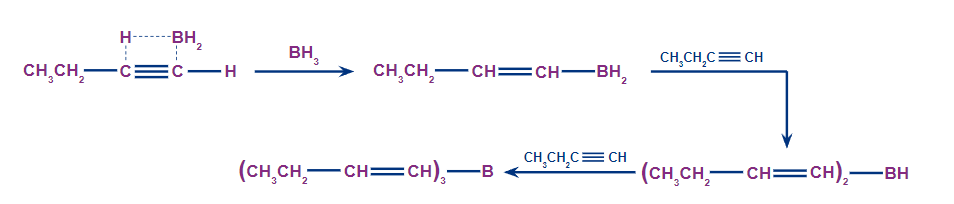
Step 2: Oxidation
Now that the trialkyl borane has been produced, the second step in the hydroboration process can begin. The boron atom is attacked in this step by the hydroperoxide ion, which is nucleophilic in nature. The R group, along with its electron bond pair is now rearranged to the adjacent oxygen atom. And simultaneously the ion of hydroxide has now been removed. This process is repeated three times to produce trialkyl borate as the product. This trialkyl borate is now treated with water to produce the required neutral alcohol. This step of the mechanism is illustrated below.
Practice Problems
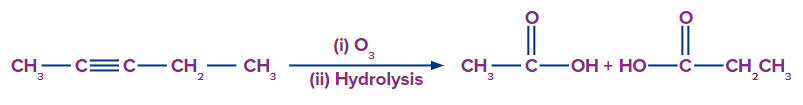
Q. 1. What are the products formed in the following reaction?
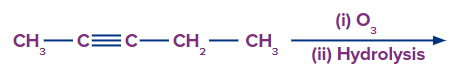
A.
B.
C.
D.
Solution: In the absence of Zn, ozonolysis followed by hydrolysis produces acid. Hence, ethanoic acid and propanoic acid are formed by oxidative ozonolysis of pent-2-yne.
Hence, option (B) is the correct answer.
Q. 2. 1 mol of 1,2-dibromopropane on treatment with X mol of NaNH2 followed by the treatment with ethyl bromide gave pent-2-yne. What is the value of X?
A. 1
B. 2
C. 3
D. 4
Solution: When 1,2-dibromopropane reacts with one mole of NaNH2, it produces propenyl bromide, which on further reaction with NaNH2 produces alkyne. Alkyne on further reaction with NaNH2 forms a conjugate base (acts as a nucleophile) that can react with ethyl bromide to form a higher alkyne. So, 1 mol of 1,2-dibromo propane will react with 3 mol of NaNH2, which on treatment with ethyl bromide will give pent-2-yne.
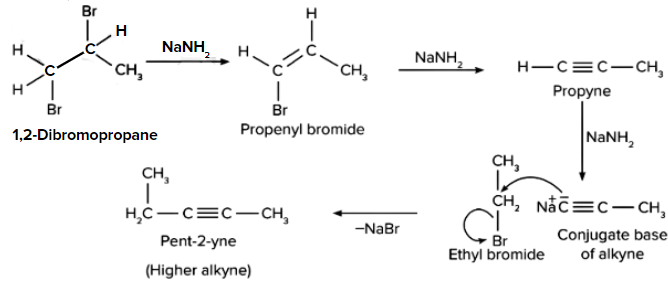
Therefore, option (C) is the correct answer.
Q. 3. In the following reaction, what are X and Y?

A. X = 1-Butyne; Y = 3-Hexyne
B. X = 2-Butyne; Y = 3-Hexyne
C. X = 2-Butyne; Y = 2-Hexyne
D. X = 1-Butyne; Y = 2-Hexyne
Solution: Ethyne, HC≡CH, on reaction with sodamide (NaNH2), forms
(conjugate base, acts as a nucleophile) which on reaction with ethyl bromide forms 1-butyne, HCC-CH2-CH3. So, X is 1-butyne.
Now, 1-butyne on reaction with NaNH2 produces a conjugate base (CH3CH2-CC-Na+), which on reaction with ethyl bromide forms , i.e., 3-hexyne. So, Y is 3-hexyne.
Hence, the correct option is (A).
Q. 4. Which of the following is product B?
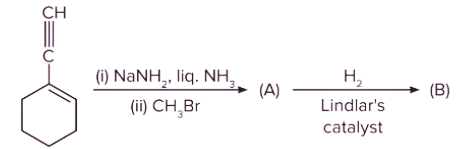
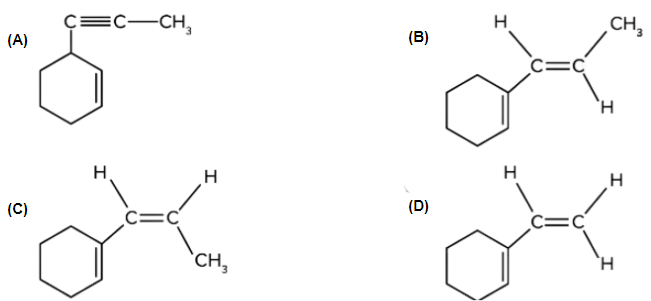
Solution: The given reactant (terminal alkyne substrate) on reaction with sodamide (NaNH2), is deprotonated and gives the conjugate base. The reaction of the conjugate base (which acts as a nucleophile) with methyl bromide (CH3Br), produces higher alkyne. The product A is shown below:
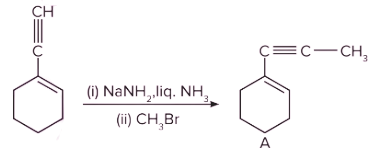
Now, A on reaction with Lindlar’s catalyst converts triple bond (−C≡C−) into cis double bond (>C=C<). Syn-addition would happen in this conversion. So, product B is as follows:
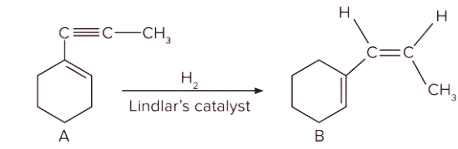
Therefore, option (C) is the correct answer.
Q. 5. Determine the products P and Q.
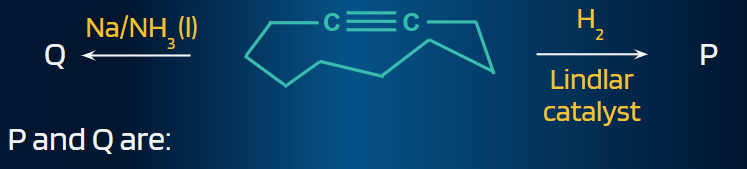
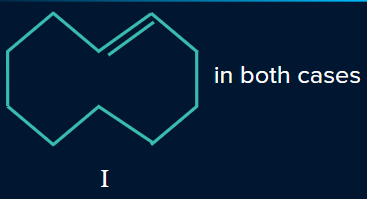
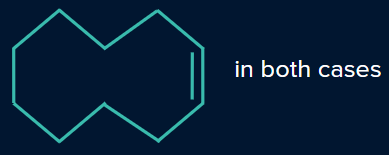
- P is I, Q is II
- P is II, Q is I
Solution:
In the presence of a Lindlar’s reagent, only cis alkenes are formed, while the Birch reduction forms a trans product.
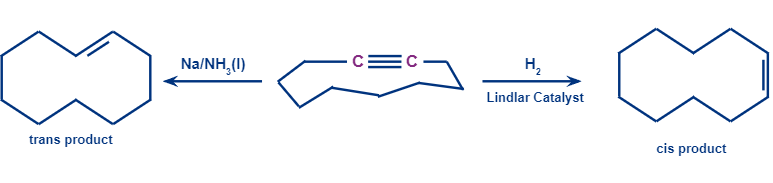
So, P will be a cis alkene, while Q will be a trans alkene.
Hence, option (D) is the correct answer.
Frequently Asked Questions
Q. 1. What type of reaction is very common for alkynes?
Answer: Alkenes and alkynes have very similar reactivity. They undergo electrophilic additions such as halogenation and hydrohalogenation. They can also be reduced with the help of a heterogeneous catalyst or oxidized using a variety of methods.
Q. 2. What is an example of an electrophilic addition reaction?
Answer: Electrophiles are compounds that lack electrons. One sigma bond and two pi bonds exist between two carbon atoms in the alkyne. These are electron-rich species that form bonds with electrophiles to give electrophilic addition reactions.
Examples include halogenation and hydration reactions.
Q. 3. Is an alkyne polar or nonpolar?
Answer: Alkynes are nonpolar, unsaturated hydrocarbons with physical properties similar to alkanes and alkenes. Alkynes dissolve in organic solvents, have little solubility in polar solvents, and are insoluble in water. In comparison to alkanes and alkenes, alkynes have significantly higher boiling points.


