-
Call Now
1800-102-2727
Qualitative Analysis-Detection of Carbon, Detection of Hydrogen, Sodium Fusion Extract, Detection of Nitrogen, Detection of Sulfur, Detection of Halogens, Detection of Phosphorus, Practice Problems & FAQs
The elements present in organic compounds are carbon and hydrogen. In addition to these, they
may also contain oxygen, nitrogen, sulphur, halogens, and phosphorus.
To precisely determine the structure of an organic compound, it is necessary to know its molecular
formula. Practical organic chemistry finds its application in deciphering the structure of an organic
compound using various experimental techniques.
An organic compound can be analyzed using ‘Qualitative analysis’ & ‘Quantitative analysis’, which
are explained below:
Table of Content
- Qualitative analysis
- Detection of Carbon and Hydrogen
- Lassaigne’s extract/Sodium fusion extract
- Detection of nitrogen
- Detection of sulphur
- Detection of halogens
- Detection of phosphorus
- Practice Problems
- Frequently Asked Questions
Qualitative Analysis
Qualitative analysis helps in the identification/detection of the elements present in an organic compound.
Detection of elements:
- Carbon and hydrogen
- Nitrogen
- Sulphur
- Halogen
- Phosphorous
Detection of Carbon and Hydrogen
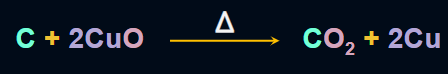
- The method for the detection of carbon and hydrogen is known as Liebig’s method.
- In this method, an organic compound is heated with CuO (cupric oxide). The carbon present
in the compound is oxidised to CO2 and the hydrogen present in the compound is oxidised
to H2O.
![]()
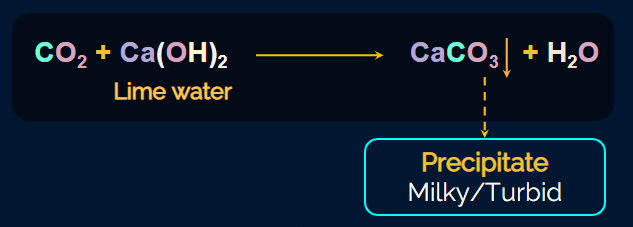
The gas formed (CO2) is tested by passing it through lime water solution (saturated solution of Ca(OH)2, then the turbidity or milkiness appears due to the formation of CaCO3
When an excess of CO2 is passed through Ca(OH)2, the milkiness disappears as calcium bicarbonate is formed, which is colourless.
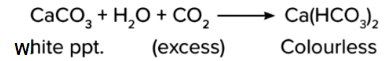
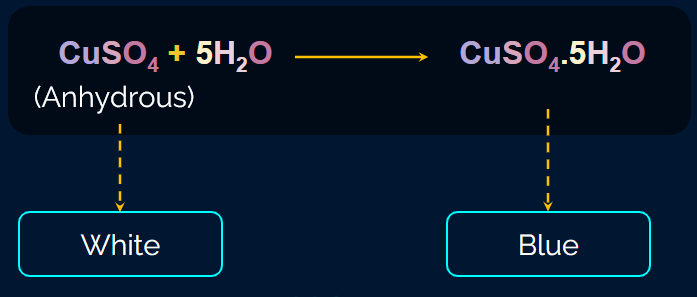
The H2O formed in the reaction is tested with anhydrous copper sulphate (white colour). In the presence of water, CuSO4 (white) becomes CuSO4.5H2O (blue colour).
Experiment Setup for Liebig’s Method
An experiment setup for Liebig’s method is given in below two parts.
Part 1:
An organic compound is mixed with CuO in a test tube, and this mixture is heated using a burner. Carbon and hydrogen are oxidised to CO2 and H2O, respectively. First, H2O is tested using anhydrous copper sulphate (white colour), which becomes blue in colour due to the formation of CuSO4.5H2O (copper(II) sulphate pentahydrate)
Part 2:
In the second part, CO2 is tested using Ca(OH)2 (lime water), where CO2 turns lime water milky. Hence, the presence of hydrogen and carbon in the given organic compound is confirmed.

Detection of other elements (nitrogen, sulphur, halogen, phosphorus)
Nitrogen, sulphur, and halogens element to be detected is converted to an ionic form, on treating with Lassaigne’s extract.
The elements in a compound are converted from covalent to ionic form by fusing an organic compound with sodium (Na) metal. Due to the high reactivity of sodium, it is used in Lassaigne’s test.
Lassaigne’s extract/Sodium fusion extract
Preparation of Lassaigne’s extract/Sodium fusion extract:
An experiment for the preparation of sodium fusion extract (Lassaigne’s extract) is given below in two parts.
Part 1:
A very small amount of dry sodium metal is taken in an ignition tube, and it is heated until it appears to be a shiny ball. Then, the organic compound that is to be tested is added to it and heated again until the ignition tube turns red hot. The contents in the ignition tube along with the tube are added to distilled water taken in a china dish. 
Part 2:
The dish is immediately covered with wire gauze to prevent it from bursting. The contents in the china dish are heated again so that they are reduced to one-third and are then filtered to obtain Lassaigne’s extract.

- Organic compounds containing covalent bonds with elements nitrogen, sulphur, and halogens are reacted with sodium to convert them into an ionic form so that they can be detected easily.
The reactions taking place in this process are:

Here, NaCN, Na2S, NaX, and NaSCN represent the ionic forms. Cyanide, sulphide, and halide of the sodium formed by fusion of sodium with the organic compound are extracted from the fused mass by boiling them with water. This extract is known as sodium fusion extract (Lassaigne’s extract).
Detection of nitrogen
The sodium fusion extract of nitrogen (containing NaCN) is heated with freshly prepared iron(II)sulphate, which gives sodium hexacyanoferrate(II). The reaction is given as:
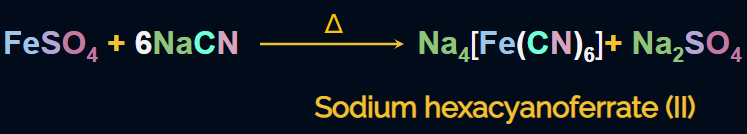
After that, on heating it with concentrated sulphuric acid, some Fe(II) ions are oxidised to Fe(III) ions. The reaction is as follows:
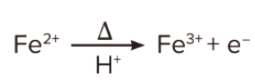
The Fe(III) ions formed react with hexacyanoferrate(II) ions to produce iron(III) hexacyanoferrate(II)
(ferriferrocyanide), which has Prussian blue colour, which confirms the presence of nitrogen.
The reaction is given as follows:
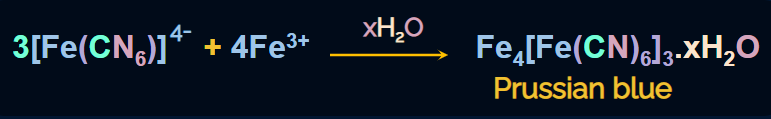
Experiment for Lassaigne’s test for nitrogen
An experiment for Lassaigne’s test for nitrogen is given below. Lassaigne’s extract is taken in a test tube and a few drops of freshly prepared FeSO4 are added to it, resulting in the formation of
- Instead of concentrated H2SO4 , we can use concentrated HNO3 , H2O2, or we can add FeCl3 directly because we need iron(III) ions for making ferriferrocyanide.
Detection of sulphur
Sulphur is detected by Lassaigne’s extract. Two major tests for the detection of sulphur in organic compounds are as follows:
- Lead Acetate Test
- Sodium nitroprusside Test
Lead acetate test
Lassaigne’s extract (containing Na2S) is acidified with acetic acid and lead acetate Pb(CH3COO)2is added to it.
A black precipitate of lead sulphide indicates the presence of sulphur. The reaction is given as follows:
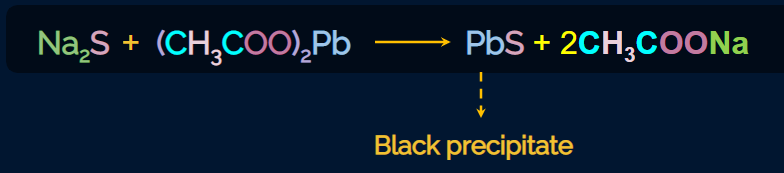
Experiment for Lead Acetate test
An experiment for the lead acetate test for sulphur using Lassaigne’s extract is given below.
Lassaigne’s extract is taken in a test tube and is acidified with acetic acid. Lead acetate is added to
this. After some time, we can observe the formation of the black precipitate of PbS, which confirms
the presence of sulphur.
Sodium nitroprusside test
On treating Lassaigne’s extract (Na2S) with sodium nitroprusside Na2[Fe(CN)5NO], the appearance of violet colour indicates the presence of sulphur. The following reaction is taking place.

Important Points:
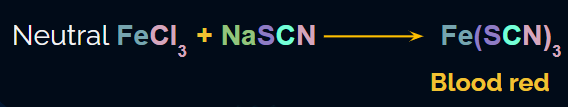
When both nitrogen and sulphur are present in the organic compound, sodium thiocyanate(NaSCN) is formed.
This sodium thiocyanate on reaction with Fe3+ will give a blood-red colour due to the formation of . It will not give a Prussian blue colour since there are no free cyanide ions.
Experiment Setup for the detection of both Nitrogen and Sulphur
An experiment when both nitrogen and sulphur are present in the compound is given below. The Lassaigne extract is placed in a test tube. It is acidified with a few drops of dilute HCl. To this, FeCl3 (Fe in +3 oxidation state) is added and can see a solution turning blood red in colour due to . This confirms the presence of nitrogen and sulphur in the organic compound.
Detection of halogens
The sodium fusion extract (NaX) is acidified with nitric acid and then treated with silver nitrate, which
lead to the formation of silver halide precipitate. There are three cases for the three halogens
(only for Cl, Br, and I), which are given as follows:

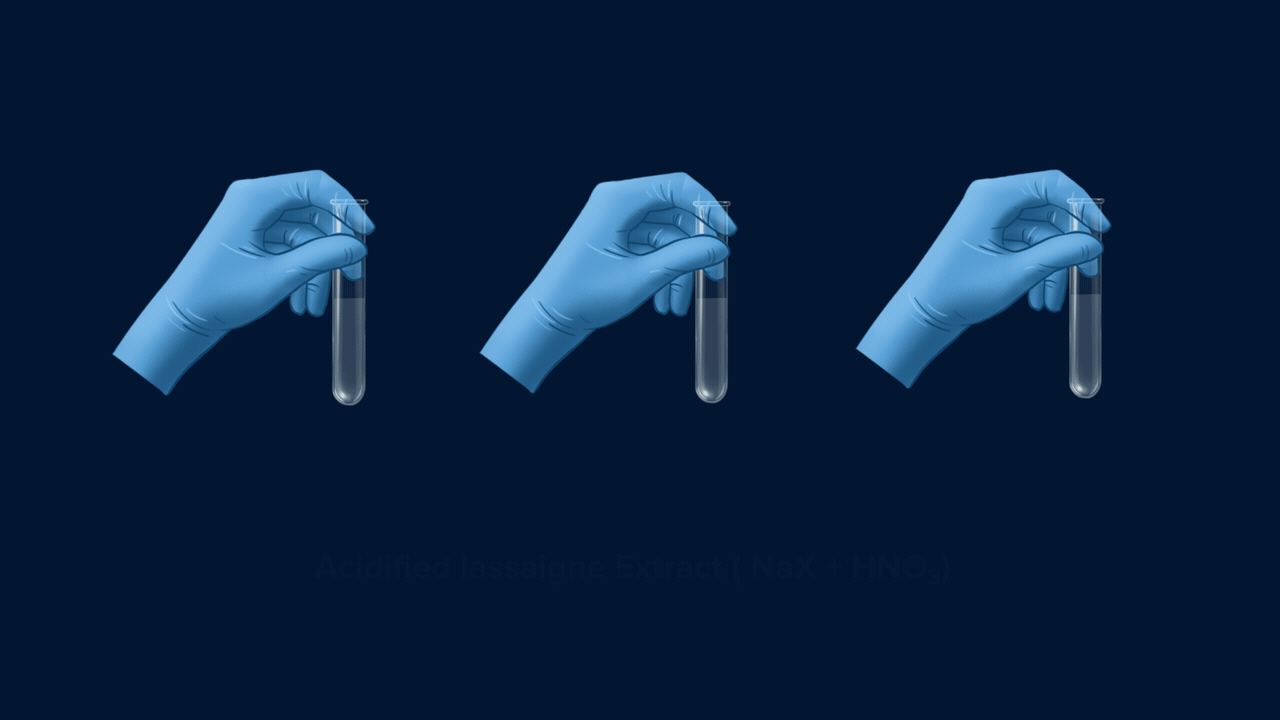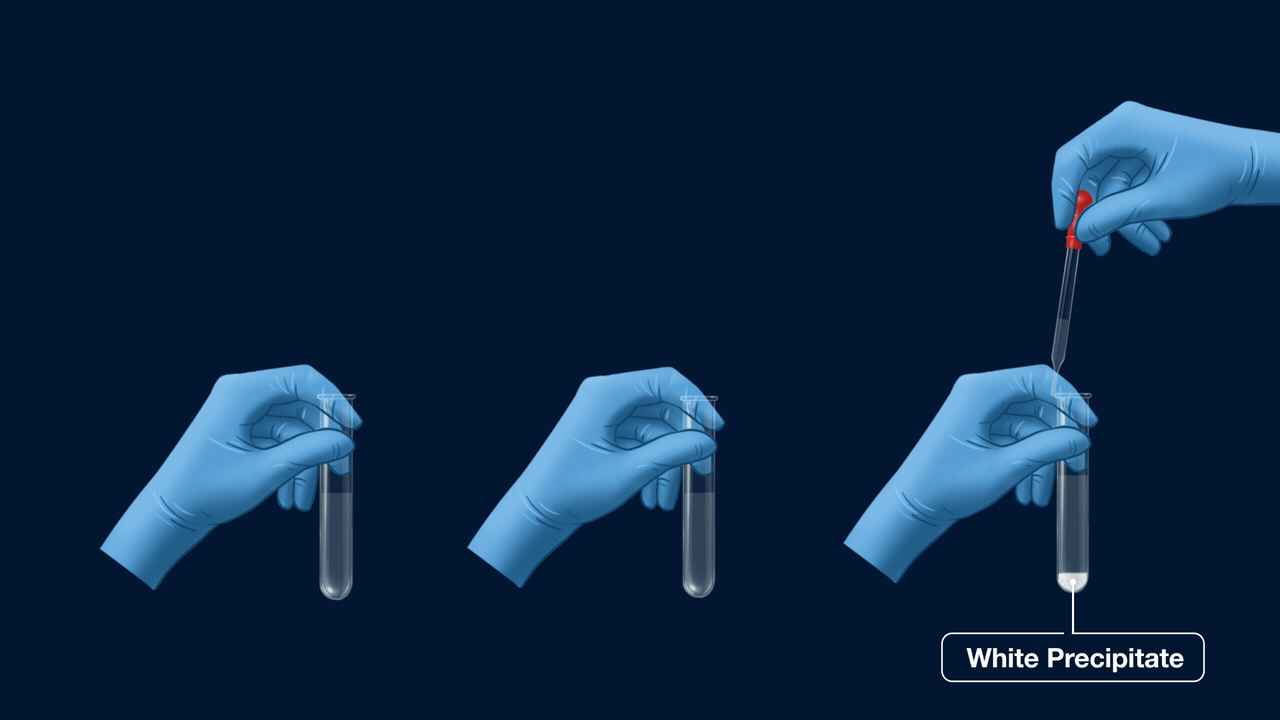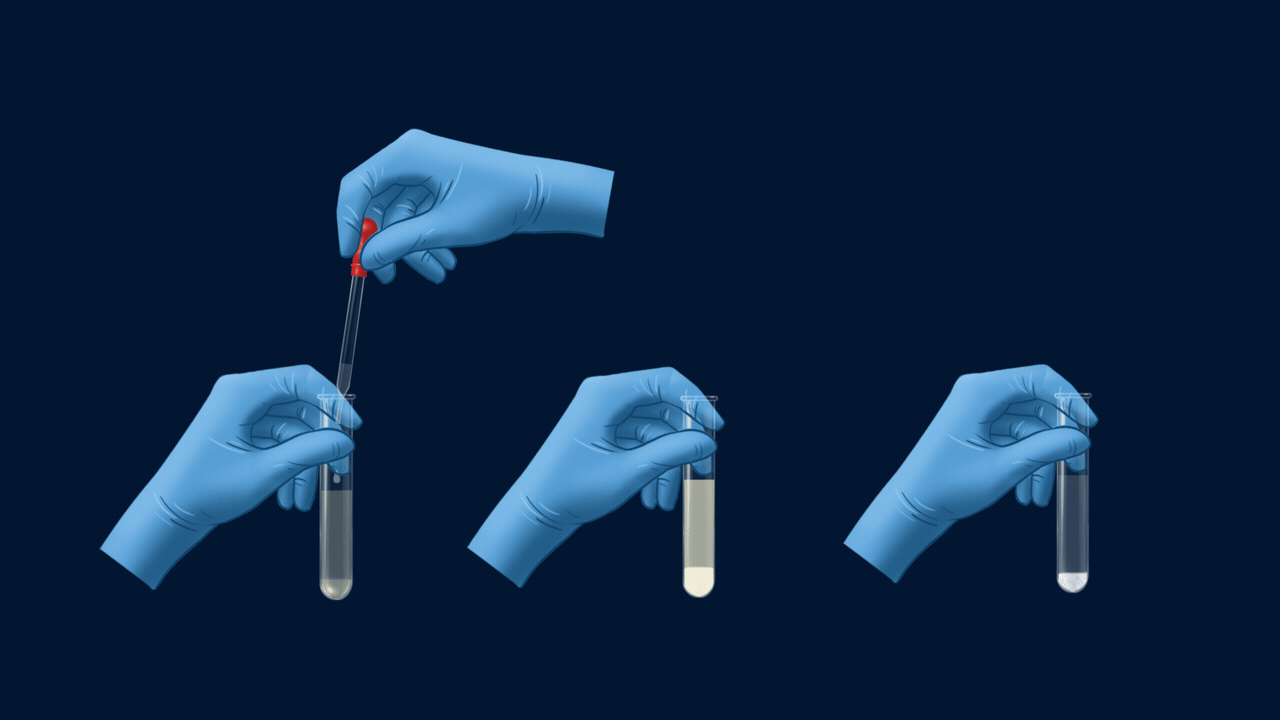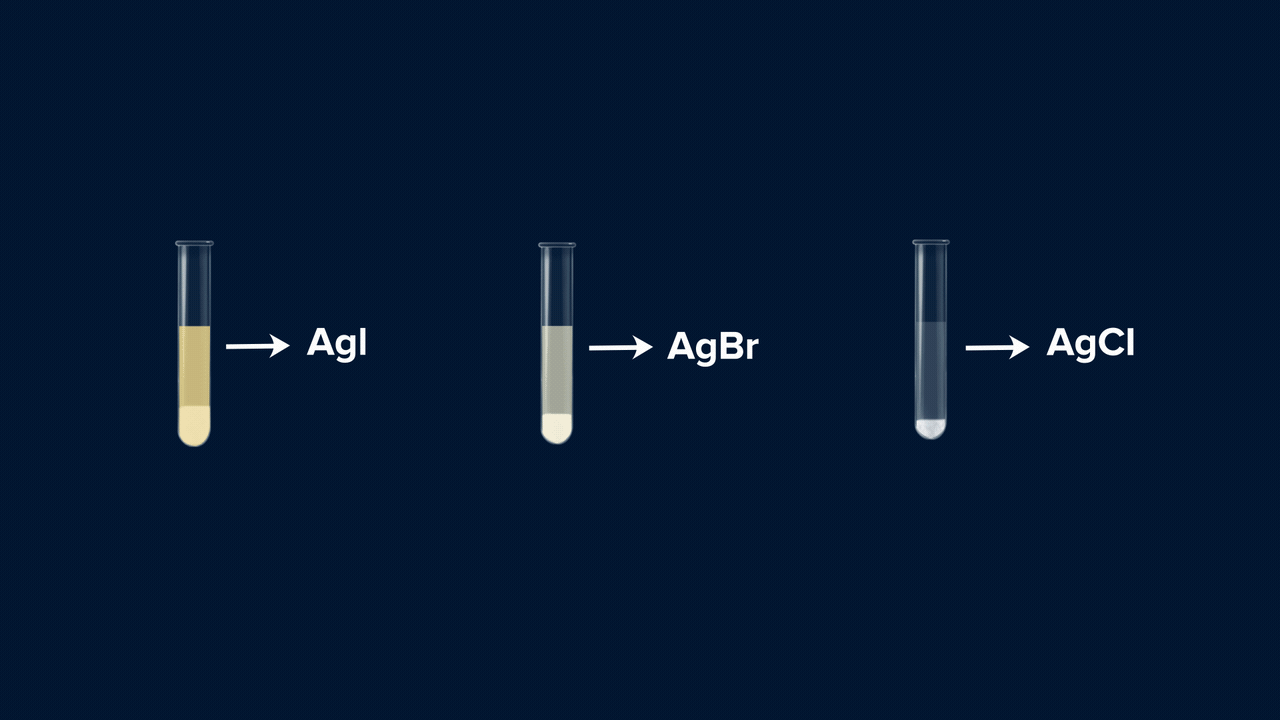
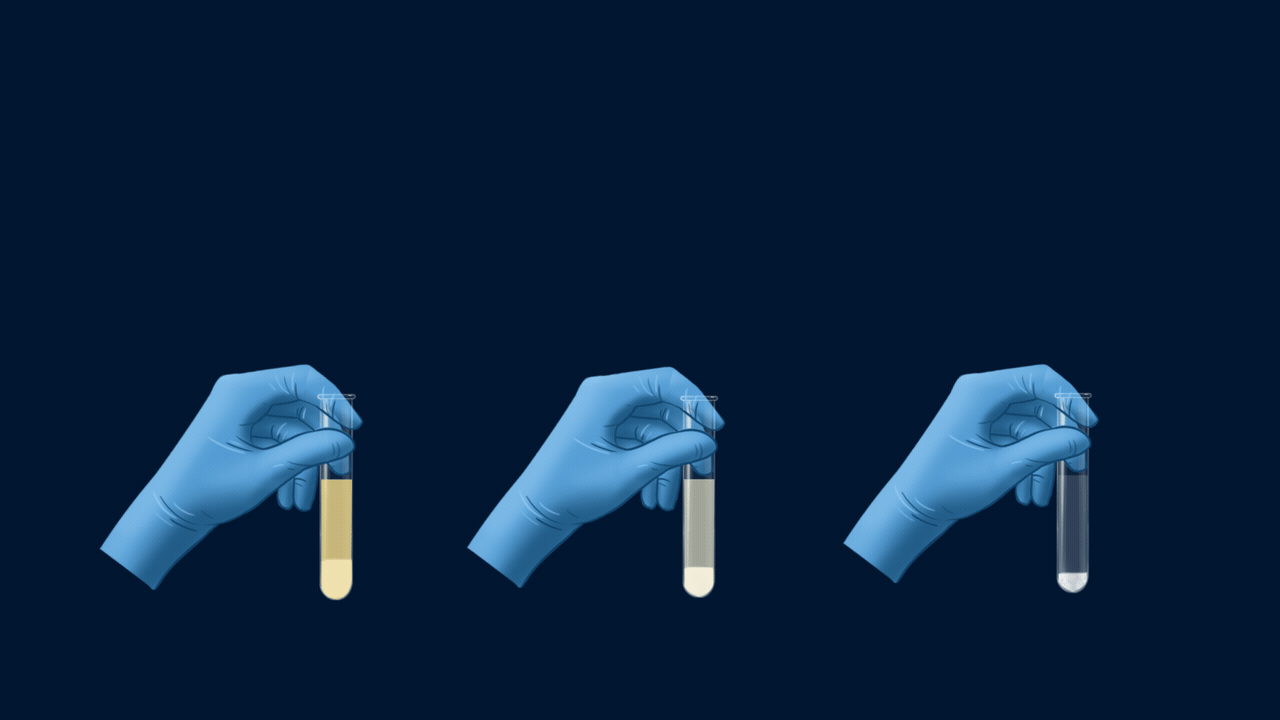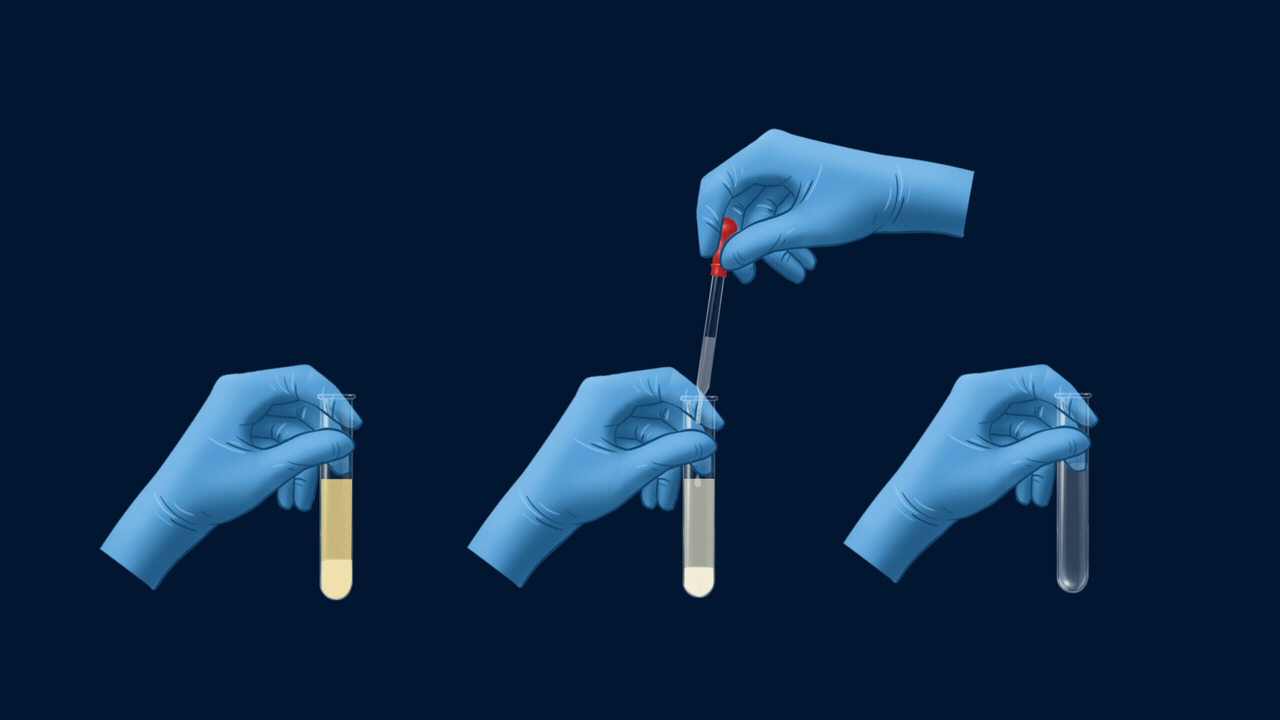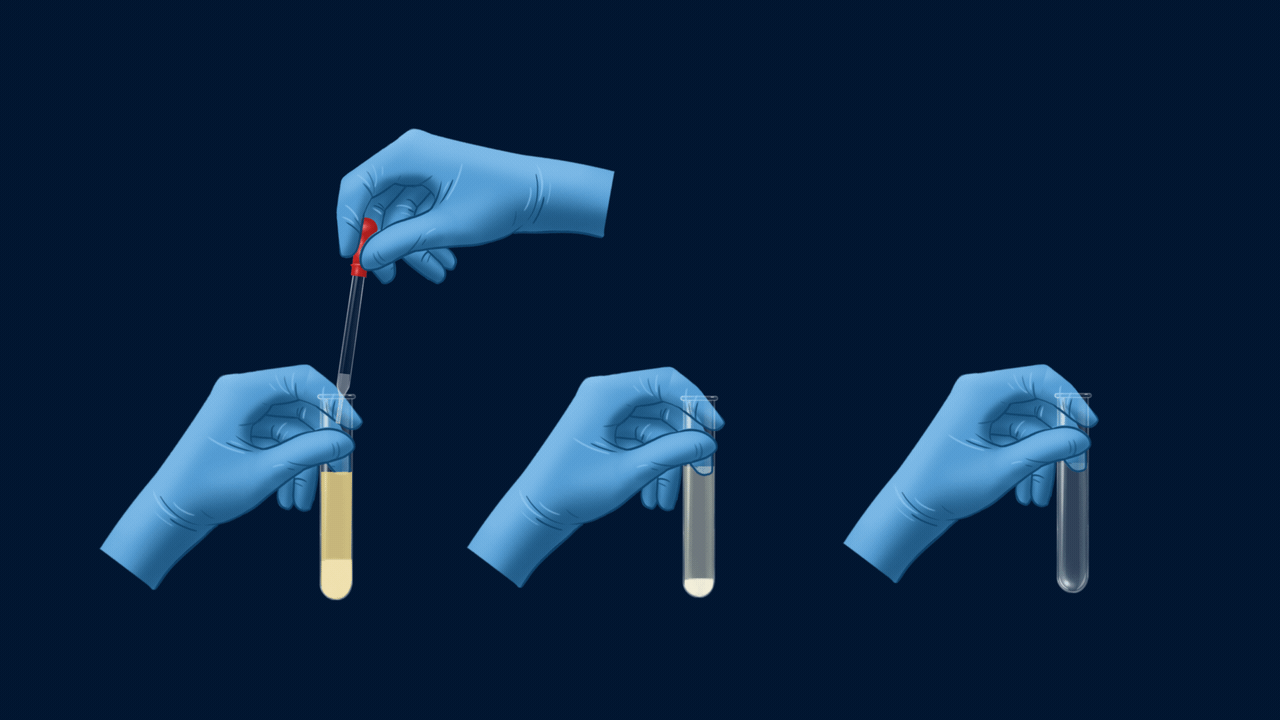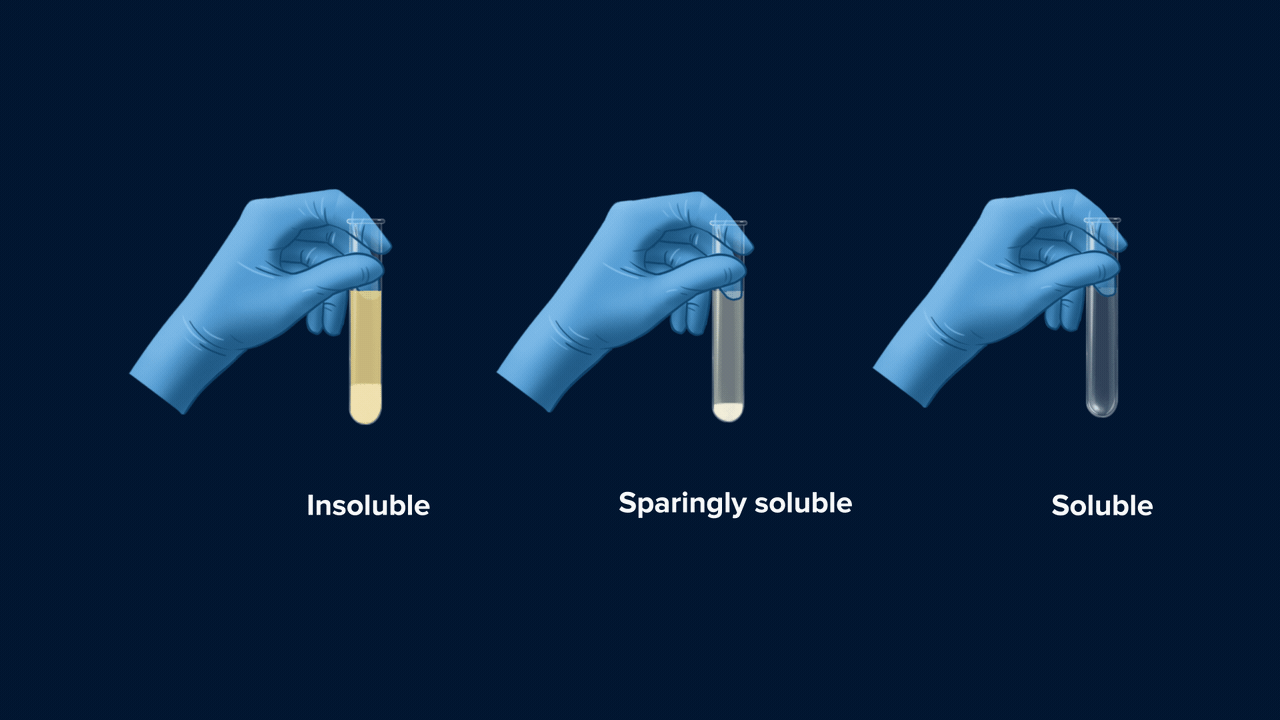
- If the colour of the precipitate (AgCl) is white and this white precipitate is soluble in NH4OH (ammonium hydroxide), then it indicates the presence of chlorine in the compound. The reaction taking place is:

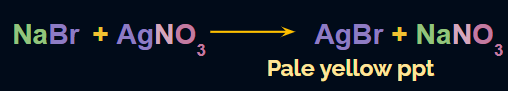
- If the colour of the precipitate (AgBr) is pale yellow (or whitish-yellow) and this pale yellow precipitate is sparingly soluble in NH4OH, then it indicates the presence of bromine in the compound. The reaction taking place is:
- If the colour of the precipitate (AgI) is yellow and this yellow precipitate is insoluble in NH4OH, then it indicates the presence of iodine in the compound. The reaction taking place is:

Experiment Setup for the test of halogens
An experiment for the test of halogens using Lassaigne’s extract. Lassaigne’s extract is taken in three different test tubes, and it is acidified using HNO3. Silver nitrate (AgNO3) is added to each of these test tubes using a dropper. The white precipitate (AgCl), which is soluble in NH4OH, indicates the presence of chlorine. The pale yellow precipitate (AgBr), which is sparingly soluble in NH4OH, indicates the presence of bromine. The yellow precipitate (AgI), which is insoluble in NH4OH, indicates the presence of iodine.
Part 1: Formation of Precipitate
Part 2: Solubility of Precipitate in Ammonium Hydroxide
Important Points:
- The presence of nitrogen or sulphur interferes with the detection of halogen using Lassaigne’s extract because both are precipitated into AgCN and Ag2S, respectively.
- These should be removed by boiling the sodium fusion extract with concentrated nitric acid to decompose sodium cyanide and/or sodium sulphide formed during Lassaigne’s test.
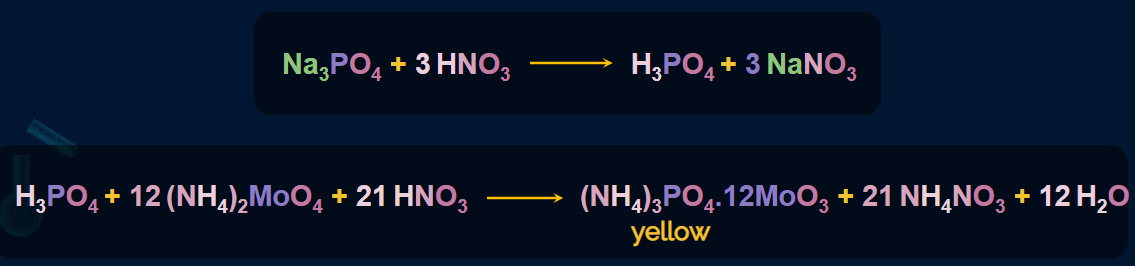
Detection of phosphorus
An oxidising agent, such as sodium peroxide, is used to heat the organic compound.
The phosphorus present in the compound is oxidised to phosphate ().
After boiling with nitric acid, the solution is treated with ammonium molybdate.
A yellow colouration or precipitate (ammonium phosphomolybdate) indicates the presence of phosphorus.
Experiment setup for the test of Phosphorous
An experiment for the test for phosphorus is given below. First, the organic compound is taken
in a test tube and it is heated on a burner by adding concentrated HNO3. Due to this, all the
phosphorus is oxidised into phosphate. Then, ammonium molybdate is added to this test tube.
Due to this, a yellow colouration or precipitate (ammonium phosphomolybdate) is formed. This
indicates the presence of phosphorus.

Practice Problems
Q. 1. The Prussian blue colour obtained during the test of nitrogen by Lassaigne’s test is due to the formation of which of the following?
A.
B.
C.
D.
Answer: A
Solution: In Lassaigne’s test for nitrogen, the formation of a Prussian blue colour confirms the presence of nitrogen. This Prussian blue colour is due to the formation of iron(III) hexacyanoferrate(II), , also known as ferriferrocyanide.
Q. 2. In Lassaigne's test, the organic compound is fused with a piece of sodium metal in order to:
A. Increase the ionisation of a compound
B. Decrease the melting point of a compound
C. Increase the reactivity of a compound
D. Conversion of covalent compounds into ionic compounds
Answer: D
Solution: The Lassaigne's test is used for detecting halogens, nitrogen, and sulphur in organic compounds. The organic compounds are covalently bonded to these elements. They must be converted into their ionic forms in order to be detected. This is done by combining the organic compound with sodium metal. The ionic compounds formed during the fusion are extracted in an aqueous solution and can be used for the detection of sulphur, nitrogen and halogens by simple chemical tests. The extract is called sodium fusion extract or Lassaigne's extract.
Q. 3. Which of the following elements in an organic compound can be detected using ammonium molybdate?
A. Carbon
B. Nitrogen
C. Phosphorous
D. Sulphur
Answer: C
Solution: Ammonium molybdate is used for the detection of phosphorus in organic compounds.
The compound is heated with an oxidising agent (sodium peroxide) so that the phosphorus present in the compound is oxidised to phosphate. The solution is boiled with nitric acid and then treated with ammonium molybdate. A yellow colouration or precipitate indicates the presence of phosphorus.
Q. 4. The Lassaigne’s extract is boiled with concentrated HNO3 before testing for halogens because:
A. AgCN is soluble in HNO3
B. Silver halides are soluble in HNO3
C. NaCN and Na2S are decomposed by HNO3
D. Ag2S is soluble in HNO3
Answer: C
Solution: The Lassaigne’s extract is boiled with concentrated HNO3 before testing for halogens because the cyanide and sulphide ions present in the extract will interfere in the detection of halogens.
These ions should be removed by boiling the sodium fusion extract with concentrated nitric acid which will decompose the cyanide or sulphide.
Q. 5. Lassaigne's test for the detection of nitrogen will fail in the case of
A.
B.
C.
D.
Answer: C
Solution: Lassaigne's test is used to determine the presence of N in organic compounds. But NH2NH2 is an inorganic compound. No carbon is present in this compound thus, it will give a negative Lassaigne's test.
In Lassaigne's test
Na + C + N → NaCN
Thus carbon must be present for the formation of NaCN which is further used in the test.
Frequently Asked Questions-FAQs
Q. 1. What are the two tests used for the detection of Sulphur in organic compounds?
Answer: There are two types of tests used to detect sulphur. Lead Acetate Test and Nitroprusside test are used for the detection of Sulphur in organic compounds.
Q. 2. Can we use a different acid instead of sulphuric acid in Lassaigne’s test for nitrogen?
Answer: Yes, In Lassaigne’s test for nitrogen, we can use concentrated nitric acid and also we can use hydrogen peroxide or we can directly use ferric chloride.
Q. 3. Why does the Ferric thiocyanate complex will not give a Prussian blue colour?
Answer: In the detection of both nitrogen and sulphur-containing compounds, A complex ferric thiocyanate is formed which is Blood red in colour. It will not give a Prussian blue colour due to the absence of free cyanide ions.
Q. 4. Name the complex formed in the detection of Phosphorous.
Answer: In the detection of phosphorous by using sodium fusion extract, a yellow precipitate of ammonium phosphomolybdate is formed.


