-
Call Now
1800-102-2727
Preparation of Alkenes: Hydrogenation of Alkynes, Dehydrohalogenation, Dehalogenation, Dehydration, Practice Problems & FAQs
Nonstick cookware is a terrific convenience because it allows you to cook with less oil and clean-up is a breeze. Every time, perfect food is cooked and easy to clean with almost no scrubbing. Do you know what kind of material is utilized to make non-stick utensil layers?

Teflon is the answer. Teflon is a trademark name for polytetrafluoroethylene, a chemical coating (PTFE). It was invented in the 1930s to make a nonstick, non-reactive surface. It can also be used to waterproof other materials, such as wiring and fabrics.
Alkenes are used to make Teflon. Then, we need to understand how alkenes are made.
Let’s have a closer look at the preparations of alkenes!
Table of Content
- Alkene
- Classification of Alkenes
- Hydrogenation of Alkynes
- Dehydrohalogenation of Alkenes
- Dehalogenation of vicinal dihalides
- Dehydration of Alcohols
- Practice Problems
- Frequently asked questions
Alkene:
Alkenes are unsaturated hydrocarbons containing at least one double bond. The general formula for alkenes is CnH2n. They are also known as olefins (oil-forming).

Classification of alkenes:
Alkyl groups connected to the sp2 hybridized carbon atoms of alkenes alter the stability of the double bond. Alkenes' chemical reactivity can also be affected by the number of alkyl groups attached to the sp2hybridized carbon atoms. As a result, alkenes can be classed based on how many alkyl groups are attached to the C=C structural unit.
A single alkyl group is attached to the sp2 hybridized carbon atom of the double bond in monosubstituted alkenes. At the end of the carbon atom chain, a terminal alkene bears a double bond. Two, three, or four alkyl groups are linked to the carbon atoms of the double bond in disubstituted, trisubstituted, and tetrasubstituted alkenes, respectively.
|
Types |
Formula |
|
Monosubstituted |
RCH=CH2 |
|
Disubstituted |
RCH=CHR, CH2=CR2 |
|
Trisubstituted |
RCH=CR2 |
|
Tetrasubstituted |
CR2=CR2 |
Relative Reactivity of Hydrocarbons:
Alkenes and alkynes have weak π bonds containing loosely held electrons, which makes alkenes/alkynes reactive towards electrophiles. Electrophiles are electron-loving species. So, alkenes and alkynes show electrophilic addition reactions.
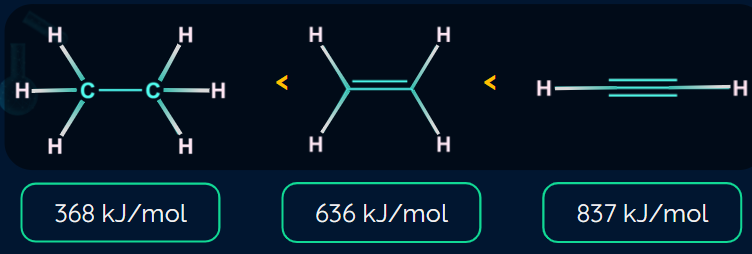
Bond Strength of Hydrocarbons:
A carbon-carbon triple bond (837 KJ mol-1) is stronger than a carbon-carbon double bond (636KJ mol-1), which is stronger than a carbon-carbon single bond (368 KJ mol-1).
Preparation of Alkenes:
Generally, alkenes can be prepared by using the following methods:
- Hydrogenation of Alkynes
- Dehydrohalogenation of Alkenes
- Dehalogenation of vicinal dihalides
- Dehydration of Alcohols
Hydrogenation of Alkynes:
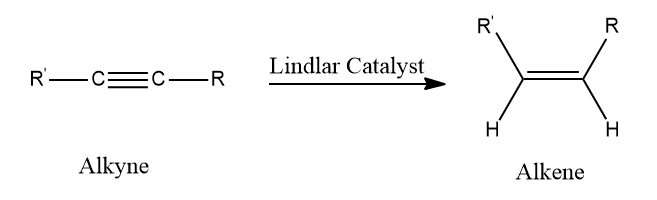
- Lindlar’s Catalyst:
Reagents: H2/Pd, CaCO3, quinoline
Poisoned palladium catalyst: It is composed of powdered calcium carbonate coated with palladium and poisoned with quinoline to reduce its catalytic activity so that a complete reduction of alkynes does not take place. Lindlar’s catalyst is used to carry out the partial reduction of alkynes to alkenes. Poisoning deactivates the catalytic activity to an extent and the reduction of the alkyne is restricted to the formation of an alkene.
Note: In the presence of Lindlar’s reagent, only cis alkenes are formed.
Example: Reduction of Alkynes using Lindlar’s Catalyst

- Birch Reduction:
The conversion of alkyne to alkene using Na /liquid NH3 in the presence of EtOH is known as the Birch reduction.
Note: This is a trans addition and alkynes give trans alkenes in the Birch reduction.
Example:
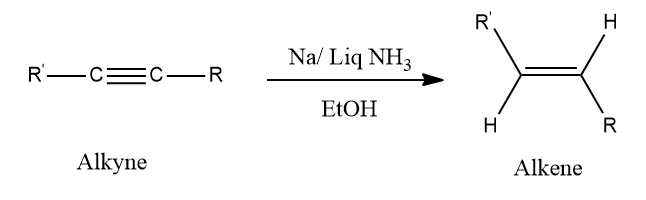
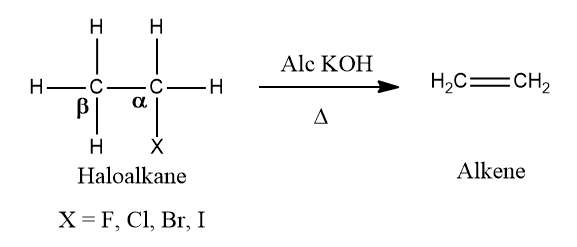
Dehydrohalogenation of Alkenes:
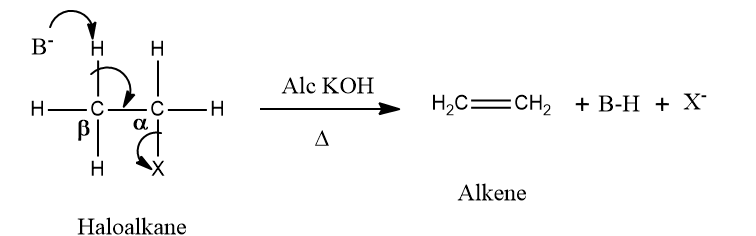
Alkenes can be prepared from alkyl halides by dehydrohalogenation, which means the elimination of HX. The reagent used is alcoholic KOH and β-Hydrogen is abstracted and is, therefore, known as the β-elimination reaction. The hydrogen opposite to the halogen atom attached to the β-carbon (carbon atom next to the carbon to which halogen is attached) is removed. Hence, it is also known as anti-elimination.
Example:
Mechanism:
The alkoxide ion sourced from the alcoholic KOH acts as a strong base. It attacks the β-H atom, which is slightly acidic in nature and separates it from the alkyl halide molecule. The electrons shared by the broken hydrogen‐carbon bond are attracted towards the ⍺-carbon atom, which is slightly electron-deficient due to being attached to the halogen atom. As these electrons approach the ⍺-carbon atom, the halogen-carbon bond breaks, leading to the formation of the double bond.
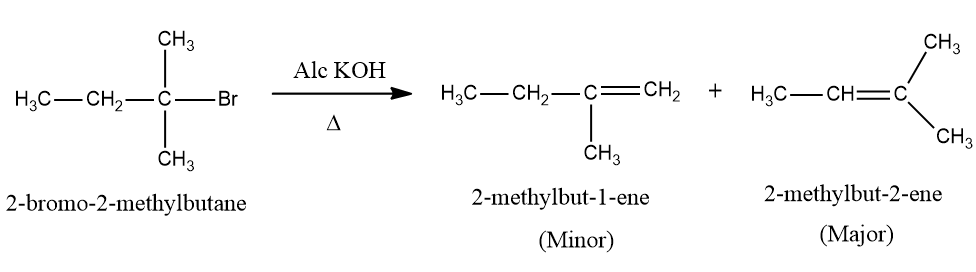
Saytzeff or Zaitsev rule:
In most elimination reactions, where there are two or more possible products, the predominant product will be the one with the highly substituted double bond.
Example:
Dehydrohalogenation of RX:
Reactivity: R−I > R−Br > R−Cl > R−F. This is because iodide is better leaving group due to its large size.
Rate of elimination:
The rate of elimination reaction directly depends upon the stability of alkene.
Rate: Tertiary alkyl halide > Secondary alkyl halide > Primary alkyl halide
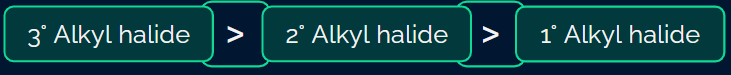
This is because tertiary halides have more number of β-hydrogens as compared to secondary and primary halides.
Dehalogenation of vicinal dihalides:
Alkenes are prepared from vicinal dihalides by dehalogenation, i.e., elimination of X2. The reagent used is NaI in acetone or Zn in the presence of acetic acid or ethanol or Zn/Δ. It is expected that this reaction proceeds through the E2 mechanism.
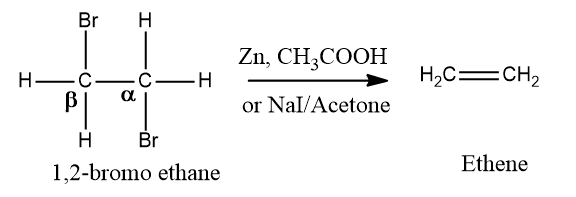
Mechanism:
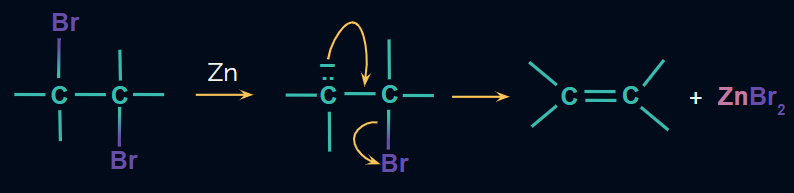
Dehydration of Alcohols:
Alkenes can be prepared from alcohol by acidic dehydration, i.e., the elimination of H2O in the presence of acid. The reagent used is conc. H2SO4 and heat. β-H is removed and therefore, it is known as the β-elimination reaction. The reaction proceeds through the E1 mechanism involving the formation of carbocation, so rearrangement may take place.
Reaction:
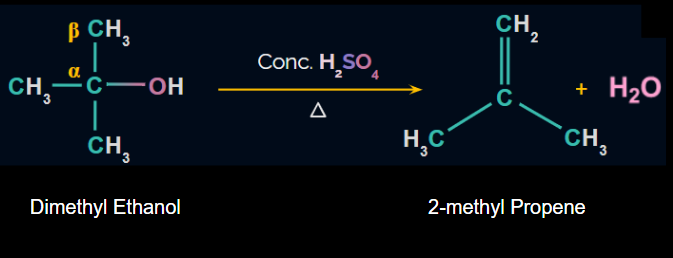
Mechanism:
Step 1: Formation of a protonated alcohol
Due to the presence of two lone pairs of electrons on oxygen, alcohols act as weak bases. Therefore, they react with strong mineral acids (H2SO4) to form a protonated alcohol. Protonation of alcoholic oxygen facilitates the elimination of water molecules.
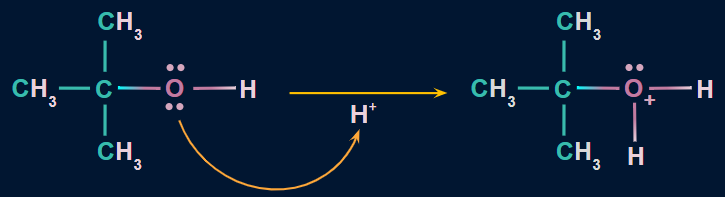
Step 2: Formation of carbocation
In this step, the C−O bond breaks with the elimination of a water molecule to form carbocation. This is the slowest step. Hence, it is considered as the rate-determining step.
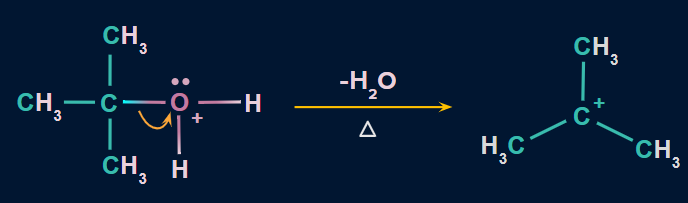
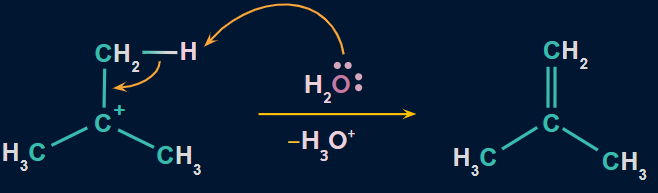
Step 3: Formation of alkene
Here, water attacks the proton of the carbon atom adjacent to the carbocation, breaking the existing C-H bond to form C=C.
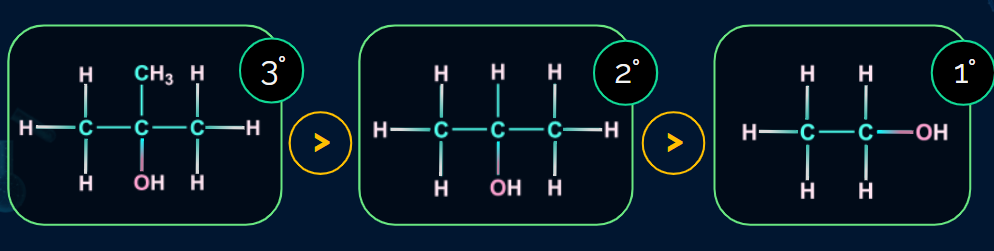
Order of reactivity of alcohols towards dehydration:
Practice Problems:
Q1. Will the compound given in the following reaction give a β-elimination product?
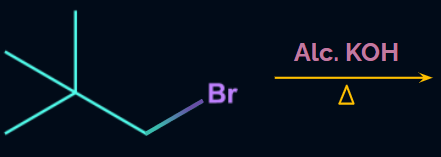
- Yes
- No
- May be
- It will go under - elimination
Answer: (B)
Solution:
The presence of β-hydrogen is a necessary condition for the dehydrohalogenation reaction (β-elimination reaction).
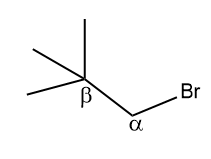
Since the given compound does not contain any β-hydrogen, it does not undergo a dehydrohalogenation reaction. Hence, the correct answer is option (B).
Q2. What is the increasing order of rate of dehydrohalogenation reaction? Given (I) - first structure, (II) - second structure, (III)- third structure.
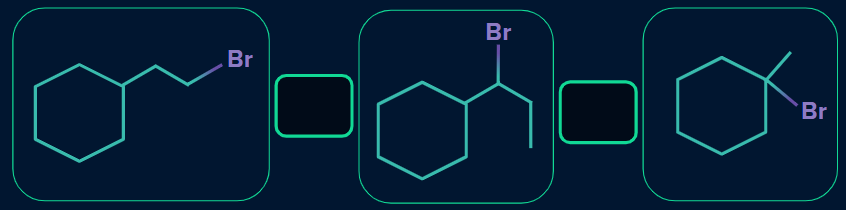
- (I) < (II) < (III)
- (I) = (II) = (III)
- (I) > (II) < (III)
- (I) < (II) > (III)
Answer : (A)
Solution:
We know that the rate of alkyl halide towards dehydrohalogenation reaction is as follows:
Tertiary alkyl halide > Secondary alkyl halide > Primary alkyl halide
(I) is the primary alkyl halide, (II) is the secondary alkyl halide, and (III) is the tertiary alkyl halide. So,
the order will be: (I) < (II) < (III).
The increasing order of rate of dehydrohalogenation reaction is: (I) < (II) < (III).
Hence the correct answer is option (A).
Q3. Find the major product of the given reaction.
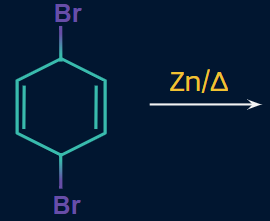
- Naphthalene
- Diphenyl
- Benzene
- All of these
Answer : (C)
Solution:
According to the mechanism, electrons will attack in the antibonding orbital of bromine and bromine will get eliminated.
Zn Zn+2 + 2e-
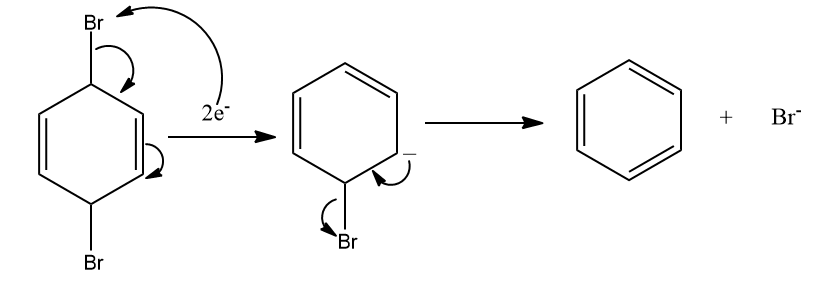
Then, delocalisation takes place and finally, another Br is eliminated, resulting in the formation of benzene as the product. Hence, the correct answer is option (C).
Q4. In the given reaction, what will be the major product A?
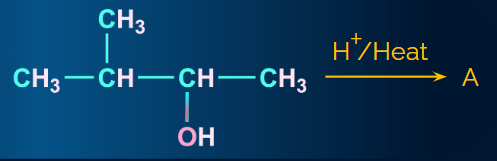
- 1-methylbut-2-ene
- 3-methylbut-1-ene
- 2-methylbut-1-ene
- 2-methylbut-2-ene
Answer : (D)
Here, secondary carbocation is formed after the elimination of water. It is converted to tertiary carbocation via a hydride shift. In the final step, deprotonation takes place to form the major alkene.
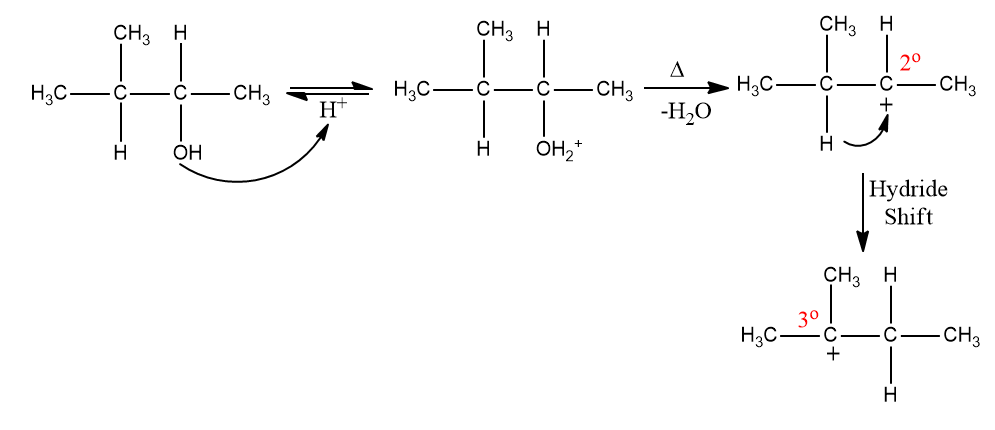
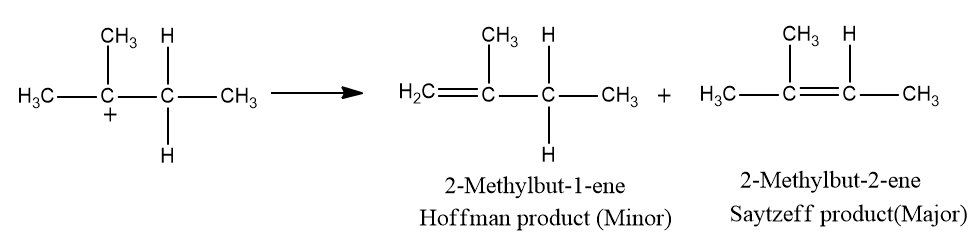
The major product formed is 2-methylbut-2-ene. Hence, the correct answer is an option (D).
Frequently asked questions:
1. What are alkenes and how do we use them?
Answer: Alkenes are unsaturated hydrocarbons containing at least one double bond. The general formula for alkenes is CnH2n. They are also known as olefins (oil-forming). Alkenes are used in a variety of ways in the manufacturing industry. They are used as starting ingredients in the production of alcohols, polymers, lacquers, detergents, and fuels.
2. Where can you find alkene?
Answer: When ethane is thermally cracked, ethene and hydrogen molecules are produced. Alkenes are used to make plastics including polyethene, PVC, polypropylene, and polystyrene. Unsaturated lipids, beta-carotene, and light transmitted through vision all contain alkene chemistry.
3. What is the meaning of the term "paraffin" when it refers to alkanes?
Answer: "Paraffin" is a Latin word that means "paraffin." Alkanes are referred to as paraffin because they have a low affinity for a general reagent. To put it another way, alkanes are deadly compounds. Only in the most extreme conditions do they react.
4. Is it possible to use alkenes as a source of energy?
Answer: If the combustion is complete, alkenes, unlike alkanes, burn quickly to produce carbon dioxide and water. They are not, however, used as fuels for two reasons. They are far too valuable to be employed in the manufacture of plastics, antifreeze, and a number of other products.


