-
Call Now
1800-102-2727
Phosphorus Trichloride (PCl3) - Structure, Preparation, Properties, Practice Problems and FAQ
We are often told to wash fresh vegetables brought from the market. In fact, no reluctance is allowed in our kitchens when it comes to cleansing the vegetables off the excess chemicals stuck to them, which might have been used while growing them on fields. Yes, even staple crops like the production of rice, wheat and many others, require a lot of insecticides and pesticide spray so that the yields are not hampered due to large-scale pest invasions and disturbance like that.
Chemicals have been infamous for their harmful effects especially now when environmental and health enthusiasts are trying to limit their usage. But no one can indeed deny the large-scale dependence that farmers, businessmen, and agriculturists have on them for benefiting crop yields.
One such precursor chemical, which is widely acclaimed for its utility in manufacturing pesticides, herbicides and insecticides, is– Phosphorus trichloride.
Let’s understand more about it here! It is indeed an integral compound of phosphorus.
TABLE OF CONTENTS
- Phosphorus Trichloride - Introduction
- Phosphorus Trichloride - Structure
- Phosphorus Trichloride - Preparation
- Phosphorus Trichloride - Physical Properties
- Phosphorus Trichloride - Chemical Properties
- Phosphorus Trichloride - Uses
- Practice Problems
- Frequently Asked Questions - FAQ
Phosphorus Trichloride - Introduction
The inorganic compound phosphorus trichloride has the chemical formula PCl3. When pure, it is a colourless liquid that is utilised in the production of phosphites and other organophosphorus compounds. It is a significant industrial chemical.
French chemists Joseph Louis Gay-Lussac and Louis Jacques Thénard created phosphorus trichloride for the first time in 1808 by burning calomel (Hg2Cl2) with phosphorus. Humphry Davy, an English scientist, created phosphorus trichloride later that year by igniting phosphorus in chlorine gas.
It is poisonous and easily releases hydrogen chloride when combined with water. Burning molten white phosphorus in dry chlorine produces phosphorus trichloride (PCl3). When phosphorus trichloride and nitric or nitrous acid come into contact, an explosion happens. A phosphorus trichloride causes immediate systemic effects by entering the circulation through the skin.
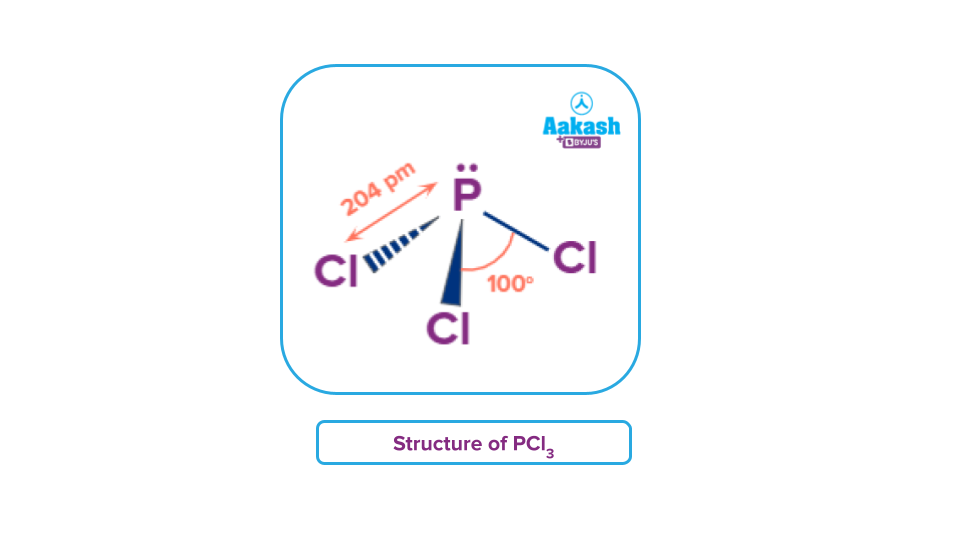
Phosphorus Trichloride - Structure
P in PCl3 is sp3 hybridised. Three of the sp3 orbitals overlap with the unhybridized p-orbitals of the chlorine atoms forming sigma-bonds, while the fourth orbital contains a lone pair of electrons. Hence, it attains a pyramidal shape.
The Cl-P-Cl bond angle is greater than usual owing to the steric crowding of the adjacent Cl atoms.
In comparison to a phosphoric acid standard, its 31P- NMR spectrum shows a singlet at about +220 ppm.
Phosphorus Trichloride - Preparation
- By passing dry chlorine gas over heated white phosphorus, phosphorus trichloride is produced.
In this process the obtained product is continuously removed so that it does not undergo further chlorination to produce phosphorus pentachloride.
- Thionyl chloride reacts with white phosphorus to produce phosphorus trichloride.
Phosphorus Trichloride - Physical Properties
- Phosphorus trichloride is a highly corrosive, as well as highly reactive, colourless liquid or sometimes a yellow fuming liquid having a very pungent and irritating smell.
- Phosphorus trichloride is an oily, colourless liquid that is highly toxic in nature.
- The boiling point of PCl3 is 349 K.
- The melting point of PCl3 is 161 K.
- The molar mass of phosphorus trichloride is 137.33 g mol-1.
- The density of phosphorus trichloride is 1.57 g cm-3.
- Its molecular geometry is trigonal pyramidal.
- It is soluble in water.
Phosphorus Trichloride - Chemical Properties
- Phosphorus trichloride on further chlorination produces phosphorus pentachloride.
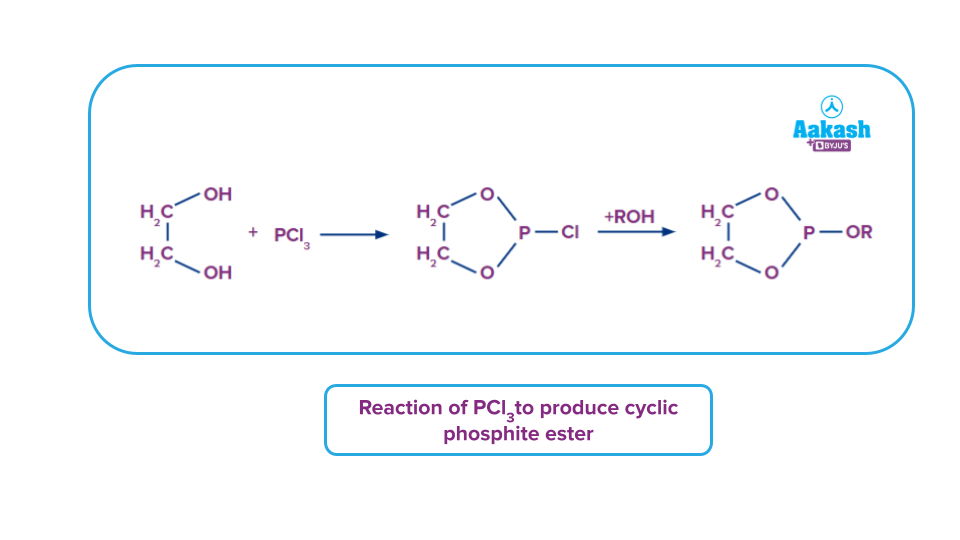
- In the presence of pyridine or dimethylaniline, PCl3 combines with diols such ethylene glycol to produce cyclic phosphorochloridate esters that react with alcohols to produce the equivalent cyclic phosphite esters.
- Phosphorus trichloride readily reacts with water and forms phosphorus acid along with hydrochloric acid.
- Phosphorus trichloride, has a lone pair and can behave both as a nucleophile and can also act as a ligand in forming coordination complexes, for example, Mo(CO)5PCl3.
- On reacting with concentrated H2SO4, chlorosulphonic acid is produced.
- The precursor of organophosphorus compounds is phosphorus trichloride.
- The Kinnear-Perren reaction takes use of this Lewis basicity to create alkyl phosphonyl dichloride (RP(O)Cl2) and alkyl phosphonate esters (RP(O)(OR')2).
- It reacts with Grignard reagents to produce substituted phosphines. For example, it forms triphenylphosphine with phenyl magnesium chloride.
- PCl3 undergoes an industrially relevant reaction known as phosphonomethylation by reacting with secondary amines and formaldehyde, producing aminophosphines.
- The most significant substitution reaction involving phosphorus trichloride is the creation of phosphites by interaction with phenols and alcohols. Phosphorus trichloride exhibits electrophilic behaviour. For instance, triphenyl phosphite is created from phenol.
- And alcohols also react in the same way only in presence of a base like tertiary amine.
- Phosphorus trichloride reacts with finely divided reactive metals to give metal chlorides.
- PCl3 on reacting with organic compounds having (-OH) replaces the hydroxyl groups with chlorine atoms and phosphorus acid is formed as a byproduct.
- It reacts with oxygen to give phosphorus oxychloride and with sulphur to give thiophosphoryl chloride.
- PCl3 is a reducing agent. Sulphur trioxide is reduced to sulphur dioxide and sulphuryl chloride to sulphur dioxide.
Phosphorus Trichloride - Uses
- As a precursor of PCl5, POCl3, and PSCl3, which are utilised in several applications, such as herbicides, insecticides, plasticizers, oil additives, and flame retardants, PCl3 is significantly used.
- Phosphorus trichloride is extensively used for making phosphorus oxychloride by oxidising it with oxygen. This is employed in the production of triphenyl phosphate and tricresyl phosphate, which are utilised as PVC plasticizers and flame retardants, respectively. They are also employed in the production of pesticides like diazinon.
- It is a significant intermediate used in the production of phosphate ester-based insecticides.
- It is used as a chlorinating agent for converting alkyl alcohols to alkyl chlorides and organic acids to organic acid chlorides and as a catalyst.
- PCl3 is the starting material for both the Wittig reaction, which produces triphenylphosphine(Ph3P), and the Horner-Wadsworth-Emmons reaction, which produces phosphite esters that can be utilised as industrial intermediates or as a starting material for alkene synthesis.
- Despite the fact that TOPO is typically manufactured using the equivalent phosphine, PCl3 may be utilised to create trioctylphosphine oxide (TOPO), a substance used as an extraction agent.
- In various direct syntheses of organic compounds, it is also employed. Although thionyl chloride often provides superior yields than PCl3, it is used to convert primary and secondary alcohols into alkyl chlorides or carboxylic acids into acyl chlorides.
- It is useful in producing important compounds like pseudohalogens and phosphonic acids.
Practice Problems
Q1. PCl3 can act as a nucleophile as well as an electrophile. Justify.
Answer: Phosphorus trichloride has the ability to behave as both nucleophile and electrophile, depending on the attacking species. Due to the single lone pair of electrons, it can donate this pair to the complex that lacks electrons and hence behaves as a nucleophile.
PCl3 can function as an electrophile as well. It can take electrons from compounds with abundant electrons because it has a vacant d-orbital, which increases its valency to 5.
Q2. Give an example to elaborate on the ligand nature of phosphorus trichloride.
Answer: Since phosphorus trichloride has a lone pair of electrons that it can donate to an electron-deficient metal centre, it forms coordination complexes. Example: .
Q3. Why during the preparation of phosphorus trichloride from white phosphorus and chlorine, the product needs to be constantly removed from the containers?
Answer: Phosphorus trichloride is produced by passing dry chlorine gas over heated white phosphorus.
In this process, the obtained product is continuously removed so that it does not undergo further chlorination to produce phosphorus pentachloride.
Q4. The molecular geometry of PCl3 is:
A. Trigonal Planar
B. Pyramidal
C. Square Planar
D. Tetrahedral
Answer: Option B)
Solution: P in PCl3 is sp3 hybridised. Three of the sp3 orbitals overlap with the unhybridized p-orbitals of the chlorine atoms forming sigma-bonds, while the fourth orbital contains a lone pair of electrons. Hence, it attains a pyramidal shape.
So, option B) is the correct answer.
Frequently Asked Questions - FAQ
Q1. How hazardous is phosphorus trichloride?
Answer: It is an extremely hazardous and corrosive chemical and needs extremely careful handling. It can severely irritate the eyes, burn skin and cause permanent damage. If inhaled it can cause shortness of breath to acute pulmonary edema. It may affect the kidneys and liver too. Exposure to around 25 ppm can be lethal and dangerous to life. Exposure-related hazards are more common for workers handling them in chemical factories.
Q2. Is PCl3 carcinogenic in nature?
Answer: According to research reports from the New Jersey Department of Health and Senior Services, it is a potent chemical and toxic too but has not been found to cause cancer in animals. So it is not yet declared to be a potential carcinogen.
Q3. How is phosphorus trichloride stored?
Answer: It must be stored under nitrogen in tightly closed containers in a well-ventilated and cool area. Also, we should avoid any exposure to moisture and other oxidising agents like perchlorates, peroxides, permanganates, sulphuric acid, nitric acid or hydrochloric acid etc.
Q4. Mention whether PCl3 ionic or covalent?
Answer: Because the P and Cl atoms share electrons in this compound (both being non-metallic), PCl3 is a covalent molecule.


