-
Call Now
1800-102-2727
Phosphorus Pentachloride (PCl5) – Introduction, Structure, Physical and Chemical Properties, Uses, Practice Problem, FAQ
“I have my own benefits, you see! But, stay away from me ‘cause I am toxic!” says Phosphorus pentachloride.
So, what should you do? Stay consciously ignorant of this chemical? Or would you try to know and understand it better?
Always remember one thing– It is very important to study even your worst enemy or your toughest competitor, just in order to retaliate in the best possible way possible, if the situation necessitates so.
Even the antidotes to the worst of poisons could be invented because scientists knew about those poisonous chemicals thoroughly and hence could come up with their remedial antidotes.

Many chemicals are toxic in nature, some are life-saving and some have dual properties in selected quantities and proportions! All of it is decided by the chemistry that a certain compound follows. At the root, it all depends on its basic configuration, structure, general physical and chemical properties as to how and where it will find its utility or its “motto of life”!
Hence, let's start the discussion about such an interesting chemical- an important chemical compound of phosphorus, that has commercial utility too besides being toxic.
TABLE OF CONTENTS
- Phosphorus Pentachloride - Introduction
- Phosphorus Pentachloride - Structure
- Phosphorus Pentachloride - Preparation
- Phosphorus Pentachloride - Physical Properties
- Phosphorus Pentachloride - Chemical Properties
- Phosphorus Pentachloride - Uses
- Practice Problems
- Frequently Asked Questions - FAQ
Phosphorus Pentachloride - Introduction
Phosphorus pentachloride is a crystalline solid that is greenish-yellow in colour and has an unpleasant smell. Water breaks it down into phosphoric acid and hydrochloric acid, which releases heat energy in the process.
Phosphoric acid
The reaction between dry chlorine and phosphorus trichloride can also be used to make it.
It is well known that phosphorus pentachloride has a salt-like structure when it is in its crystalline form and that it partially dissociates in solution, particularly in polar solvents like nitrobenzene.
As per the principle of mass action, in an environment containing chlorine gas or phosphorus trichloride, phosphorus pentachloride vaporises practically without dissociation; the presence of product shifts the dissociation equilibrium to the left.
Phosphorus Pentachloride - Structure
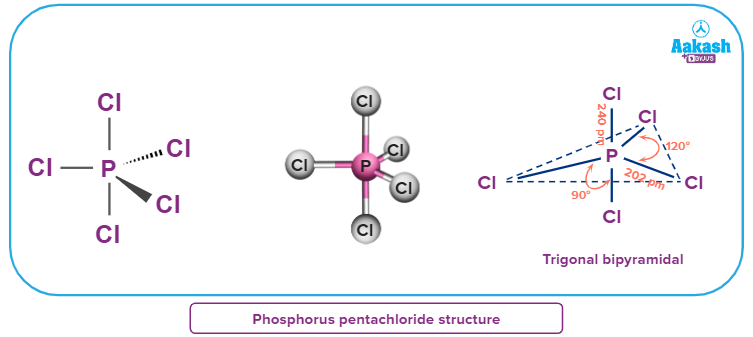
- It has a trigonal bipyramidal structure in gaseous and liquid phases.
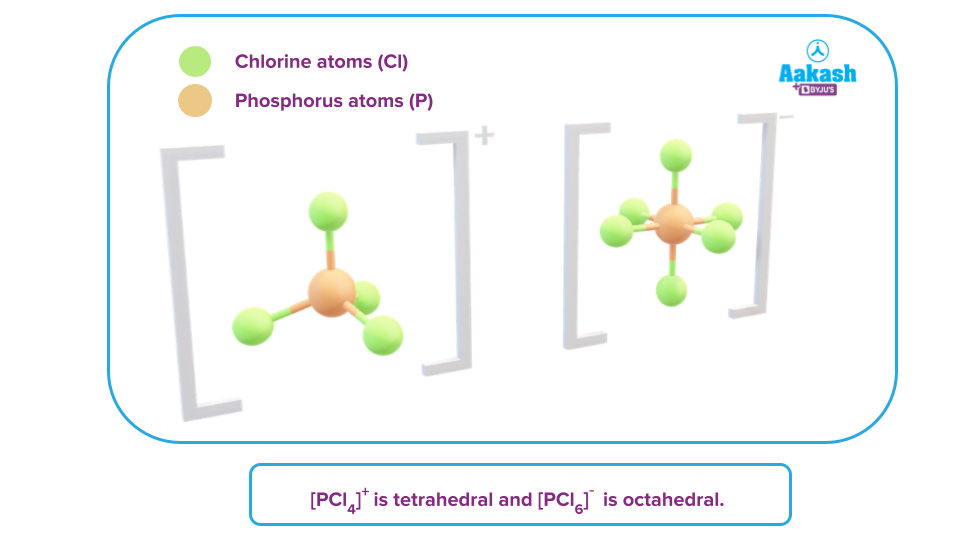
- In the solid-state, it exists as an ionic solid( ) in which the cation, , is tetrahedral and the anion, , is octahedral.
- In PCl5, phosphorus is sp3d hybridised. The 5 hybridised orbitals of phosphorus overlap with p orbitals of chlorine atoms forming 5 sigma bonds. Its molecular geometry is trigonal bipyramidal.
- Due to greater repulsion at axial positions as compared to that of the equatorial positions, the two axial bonds (242 pm) are longer and hence weaker than the equatorial bonds (202 pm).
Phosphorus Pentachloride - Preparation
Phosphorus pentachloride can be prepared in the following ways:
- Phosphorus pentachloride is prepared by reacting sulphuryl chlorides with phosphorus or phosphorus trichloride.
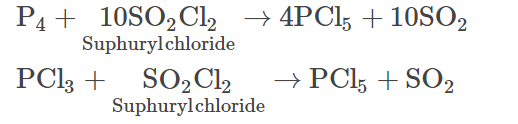
- In the laboratory, it can be prepared by passing excess dry chlorine on phosphorus trichloride.
Phosphorus Pentachloride - Physical Properties
- Phosphorus pentachloride is yellowish-white in colour with a salt-like structure in the crystalline state, and partly dissociated in solution, especially in polar solvents such as nitrobenzene.
- It is soluble in carbon tetrachloride, carbon disulfide, benzene, and diethyl ether.
- The physical properties of phosphorus pentachloride can be enlisted as follows:
|
Density |
2.1 g/cm³ |
|
Molar Mass |
208.24 g/mol |
|
Boiling Point |
166.8 °C |
|
Melting Point |
160.5 °C |
|
Chemical Formula |
PCl5 |
Phosphorus Pentachloride - Chemical Properties
The chemical properties can be visualised in terms of the various chemical reactions that it can undergo. Phosphorus exists in +5 oxidation state in PCl5.
- PCl5 sublimes on heating, but on strong heating it undergoes dissociation.
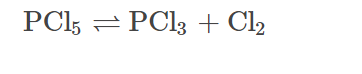
- Phosphorus pentachloride reacts violently with water. Phosphorus oxychloride is produced when there is insufficient water, and this is partial hydrolysis, whereas phosphoric acid is formed when there is an excess of water.
- On heating PCl5 with finely divided metals, the corresponding metal chlorides are produced.
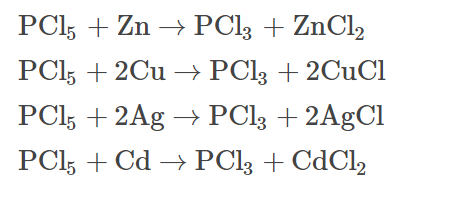
- On reaction with concentrated sulphuric acid it forms chlorosulphonic acid.
![]()
- Phosphorus pentachloride reacts with sulphur dioxide to give thionyl chloride.
![]()
- Phosphorus pentachloride reacts with organic compounds with -OH groups and replaces these groups with chlorine atoms.

- It undergoes reactions with P4O10, P4S10 and sulphur dioxide in the following manner:
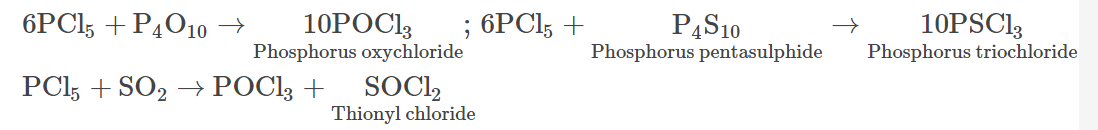
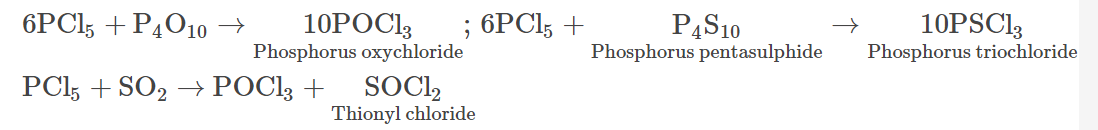
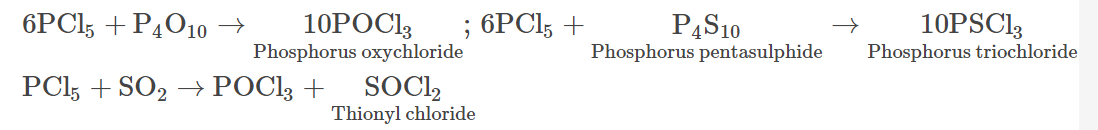
- On reacting with KF, phosphorus pentachloride forms potassium phosphorus hexafluoride.
![]()
- With some Lewis acids or chloride ion accepting species like boron trichloride and niobium tetrachloride, it produces additional compounds containing tetrachloride species [PCl4]+.
![]()
![]()
Phosphorus Pentachloride - Uses
- PCl5 acts as a chlorinating agent and as a catalyst in preparing organic chemicals, intermediates, dye-based stuff, etc.
- It acts as a catalyst in the manufacture of acetyl cellulose, which is basically the plastic film on which motion pictures are printed.
- It is widely used in the manufacture of penicillin and cephalosporin in the pharmaceutical industry.
- It is used in the production of acid chlorides.
- It acts as a catalyst for cyclisation and condensation reactions.
Practice Problems
Q. 1. Is PCl5 a Lewis acid or base?
Answer: Since acid possesses vacant orbitals in the valence shell, it is the chemical that absorbs a single pair of electrons, according to the Lewis acid concept. Electrons from other molecules can be readily accepted by the phosphorus in PCl5. As a result, it is regarded as a Lewis acid.
Q. 2. What is observed when alcohol comes in contact with PCl5?
Answer: Organic alcohols readily react with solid phosphorus pentachloride forming clouds of hydrogen chloride gas.
![]()
Q. 3. Why does phosphorus pentahalide in solid state behave as an ionic compound?
Answer: In the solid-state, phosphorus pentahalide exists as an ionic compound, in which the cation, is tetrahedral and the anion, is octahedral.
Hence, there is a strong force of attraction between these two ions, which also makes it a non-conductor of heat and electricity in solid-state.
Q. 4. Give reason for the fact that the axial bonds in PCl5 are longer than the equatorial bonds.
Answer: We know that PCl5 is sp3d hybridised, with 5 hybridised orbitals of phosphorus overlapping with p orbitals of chlorine atoms forming 5 sigma bonds. Its molecular geometry is trigonal bipyramidal. Now, the two axial P-Cl bonds are longer than the three equatorial bonds due to the higher repulsion at hub positions compared to central positions.
Frequently Asked Questions - FAQ
Q1. Why is phosphorus pentachloride more reactive?
Answer: Axial bonds exist in PCl5 in addition to equatorial bonds, which all reside in the same plane. It is longer than the other connections because it experiences more repulsion. Additionally, because these linkages are weak, they are susceptible to breaking. These factors make PCl5 particularly reactive.
Q2. Comment whether PCl5 or PCl3 is thermally stable?
Answer: PCl5 readily dissociates on strong heating and produces PCl3. Hence, PCl3 is more stable.
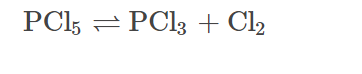
Q3. How are phosphorus pentachloride used in making lithium-ion batteries?
Answer: Phosphorus pentachloride is the precursor of lithium hexafluorophosphate (LiPF6). A salt that is frequently used in the electrolytes of lithium ion batteries is lithium hexafluorophosphate. Lithium chloride is a byproduct of the reaction between PCl5 and lithium fluoride, which results in the production of LiPF6.
Q4. What are the chemical hazards related to phosphorus pentachloride?
Answer: PCl5 is a hazardous material because it interacts with water in a severe way. Additionally, it is deadly when breathed and corrosive when it comes into touch with the skin. Phosphorus pentachloride can induce vomiting, nausea, headaches, dizziness, weakness, and irritation of the throat and nasal passages. The kidneys and liver may be harmed. Higher exposure to phosphorus pentachloride might potentially result in a medical emergency. This may result in pulmonary oedema, a fluid accumulation in the lungs that causes excruciating breathlessness.


