-
Call Now
1800-102-2727
Permanganate and Dichromatic Titration – Redox Titrations, Permanganate and Dichromatic Titration, Practice Problems and FAQ
We obsess over our smiles a lot. Teeth can grow discoloured over time, thus whitening procedures are becoming more and more common. Technically the term is commonly known as dental bleaching. Teeth are whitened using a variety of chemical preparations that contain peroxides, a procedure that is best done at a dentist's clinic.

Our toothpaste also contains peroxide, which results in teeth whitening. It is very vital to regulate the amount of chemicals entering our bodies.
So, how can we check and regulate the amount of peroxide being used in toothpaste or in the dental bleaching processes, used at the dentist’s clinic?
The answer is redox titration. Redox reactions play a crucial function in the process of dentistry. We can quickly determine how much peroxide is present in a substance by employing several redox titrations.
On this concept page, we will get to know about redox titrations, with particular emphasis on permanganate and dichromate titrations.
TABLE OF CONTENTS
- Titration
- Redox Titrations
- Potassium Permanganate Titration
- Potassium Dichromate Titration
- Practice Problems
- Frequently Asked Questions – FAQ
Titration
Titration is a method for determining a concentration of a solution by reacting it with another solution with a known concentration. In the presence of an indicator, titration is a quantitative and volumetric method for determining the concentration of an unknown solution from the concentration of a known solution.
In this process, the solution whose concentration is to be found is poured into a titration flask. The solution used in the titration flask is known as the analyte, whereas the one used in the burette is known as the titrant. Till the stoichiometric ratios of the two reagents, i.e titrant and analyte, are equal, the titration is continued. The following reaction takes place, for instance, when a strong acid like HCl is titrated against a strong base like NaOH. This is an example of an acid-base neutralisation titration.
The point at which complete neutralisation of the whole amount of HCl and NaOH present in solution takes place is known as the equivalence point.
Two distinct stages that take place throughout the titration process are the endpoint and equivalence points. An equivalence point is a point at which the number of moles of the two solutions becomes equal and neutralise each other. However, the endpoint comes just after the equivalence point. The endpoint corresponds to the immediate stage where a change in colour or intensity of the solution denotes the end of the reaction.
This is the simplest technique in which the acid-base solution is mixed with a dye that exhibits changes in colour at the endpoint, corresponding to one excess drop of either the acid/base added in the solution during the titration. These dyes are known as indicators. By displaying a colour change, the indicator aids in determining the endpoint. For instance, adding phenolphthalein to a basic solution gives it a pink colour. The endpoint is shown by the pink colour disappearing when this solution is titrated against an acid solution.
Redox Titrations
A laboratory technique for figuring out an analyte's concentration is called Redox titration. It is based on a redox reaction that occurs between the analyte and the titrant, and it occasionally involves the use of a redox indicator.
The phrase "this titration is based on a redox reaction between the analyte and the titrant" refers to the simultaneous oxidation-reduction process that occurs between the analyte and the titrant.
There are several oxidising agents that can be titrated against reducing agents like ferrous sulphate (or Fe2+ions), oxalic acid, sodium thiosulfate, potassium iodide, etc. These agents include acidified KMnO4 (Potassium permanganate), acidified K2Cr2O7(Potassium dichromate) and iodine solution.
Here are some examples of redox titrations.
- Potassium permanganate titration
- Potassium dichromate titration
- Iodine titration
Potassium Permanganate Titration
In this titration, a versatile and powerful oxidising agent, potassium permanganate (KMnO4) is used to estimate a variety of reducing agents, including Fe2+ions and oxalates ions.
Since KMnO4 is already an intense purple-coloured substance, no indicator is used in these titrations.
Thus, KMnO4 serves as a self-indicator and loses colour at the endpoint (once it is all used up) when titrated with a reducing agent. A single drop of potassium permanganate added after the reducing agent gives the solution a touch of pink colour. For instance, pink colour develops at MnO4- concentrations as low as 10-6molL-1. At the completion of the reaction, permanganate solution (KMnO4), containing Mn7+ ions is decolourised, which is indicative of the endpoint.
- In this titration, the oxidation state of manganese changes from +7 to +2 (Mn7+ to Mn2+). Hence, permanganate ions act as a strong oxidising agent as it accepts 5 electrons and gets reduced.
- Two processes are involved in this titration. First, the permanganate solution is standardised with sodium oxalate solution. Then, the analyte is titrated with potassium permanganate solution.
- Oxalate, nitrite, hydrogen peroxide, iron(II), manganese(II), and other chemical species can all be detected and quantified quantitatively using permanganometry.

Titration of MnO4- against Fe2+ions
Reduction Half-Reaction:
Oxidation Half-Reaction: ……………5
As a result, 5 moles of Fe3+ and 1 mole of Mn2+ are produced when 1 mole of MnO4- (the oxidising agent) combines with 5 moles of Fe2+ (the reducing agent).
In the net ionic form,
Overall reaction:
Titration of MnO4- against C2O42- ions
Reduction Half-Reaction: ………….2
Oxidation Half Reaction: ……………5
As a result, 10 moles of CO2 and 2 moles of Mn2+ are produced when 2 moles of MnO4- (the oxidising agent) combines with 5 moles of C2O42- (the reducing agent).
In the net ionic form,
Overall reaction:
Potassium Dichromate Titration
In redox titrations, acidified potassium dichromate (K2Cr2O7) is also employed as an oxidising agent. Compared to KMnO4, it is a weaker oxidising agent. However, unlike KMnO4, it does not function as a self-indicator because these titrations do not result in a noticeable auto-colour shift (as with MnO4-, titrations). Indicators like diphenylamine, N-phenylanthranilic acid, and the external indicator potassium ferricyanide, K4[Fe(CN)6], are frequently used in these titrations.
For example, shortly after the equivalence point or at the endpoint, K2Cr2O7 oxidises the indicator diphenylamine to produce an intense blue colour that indicates the endpoint.

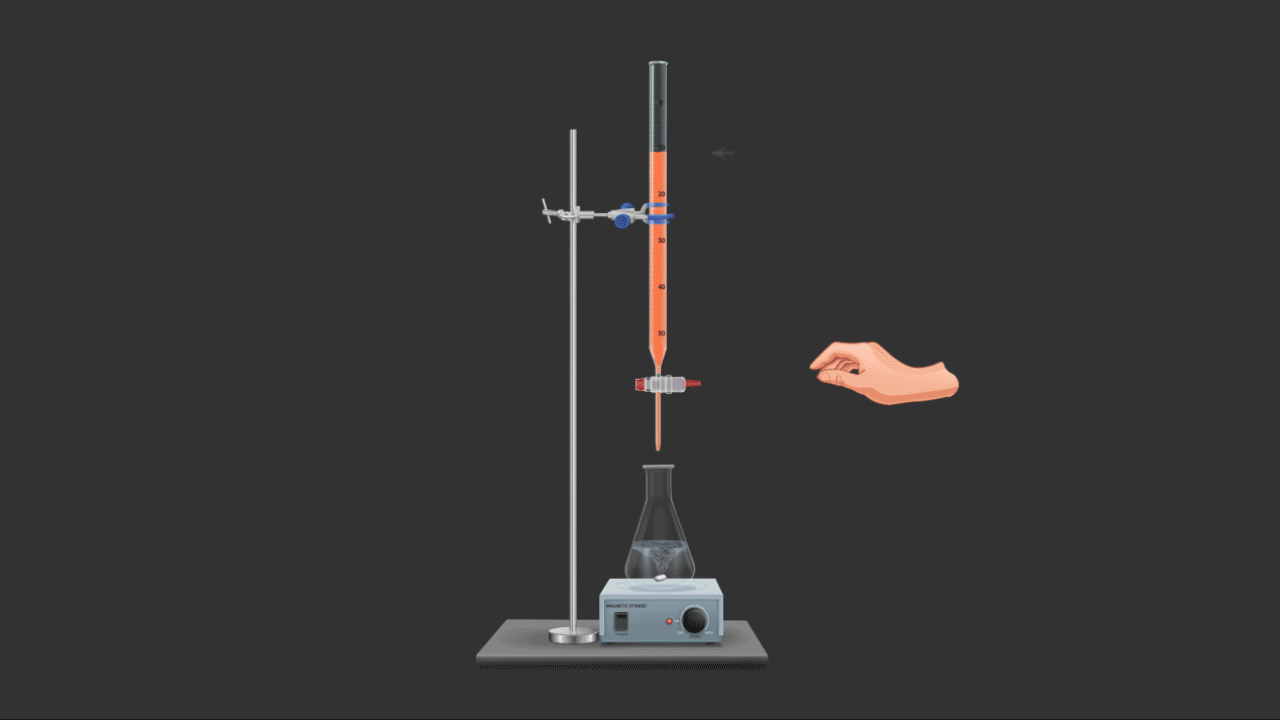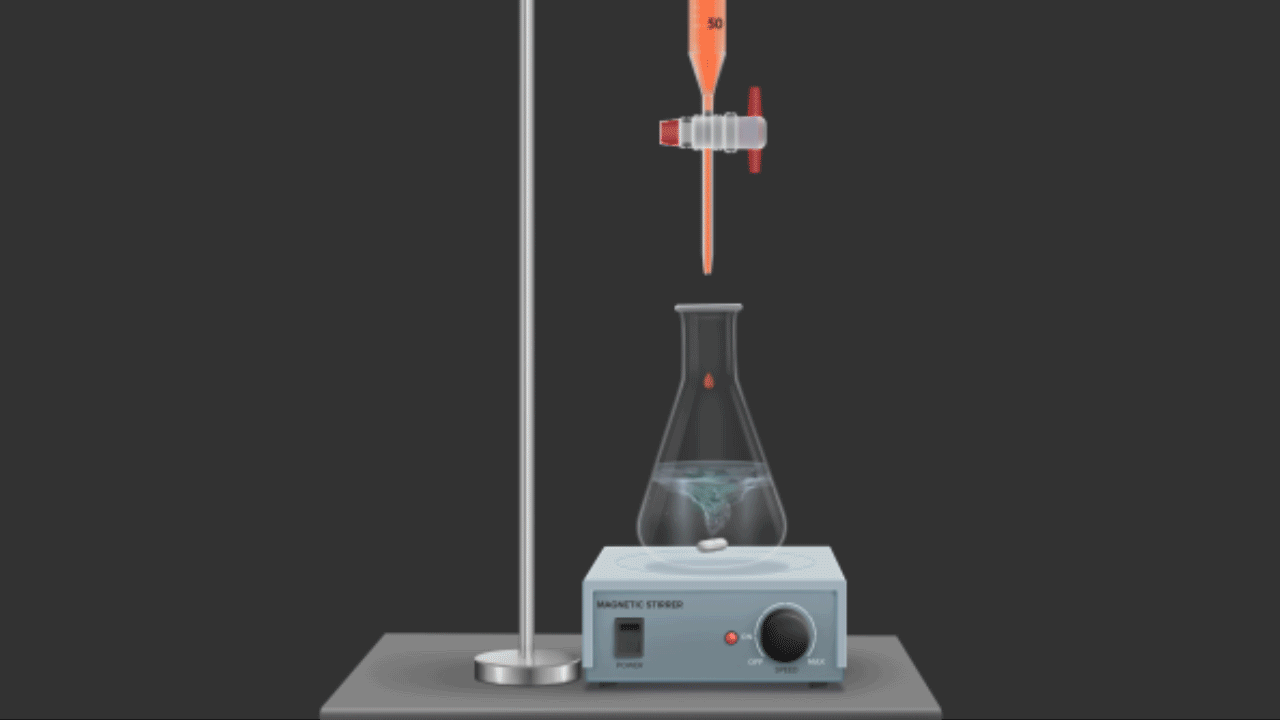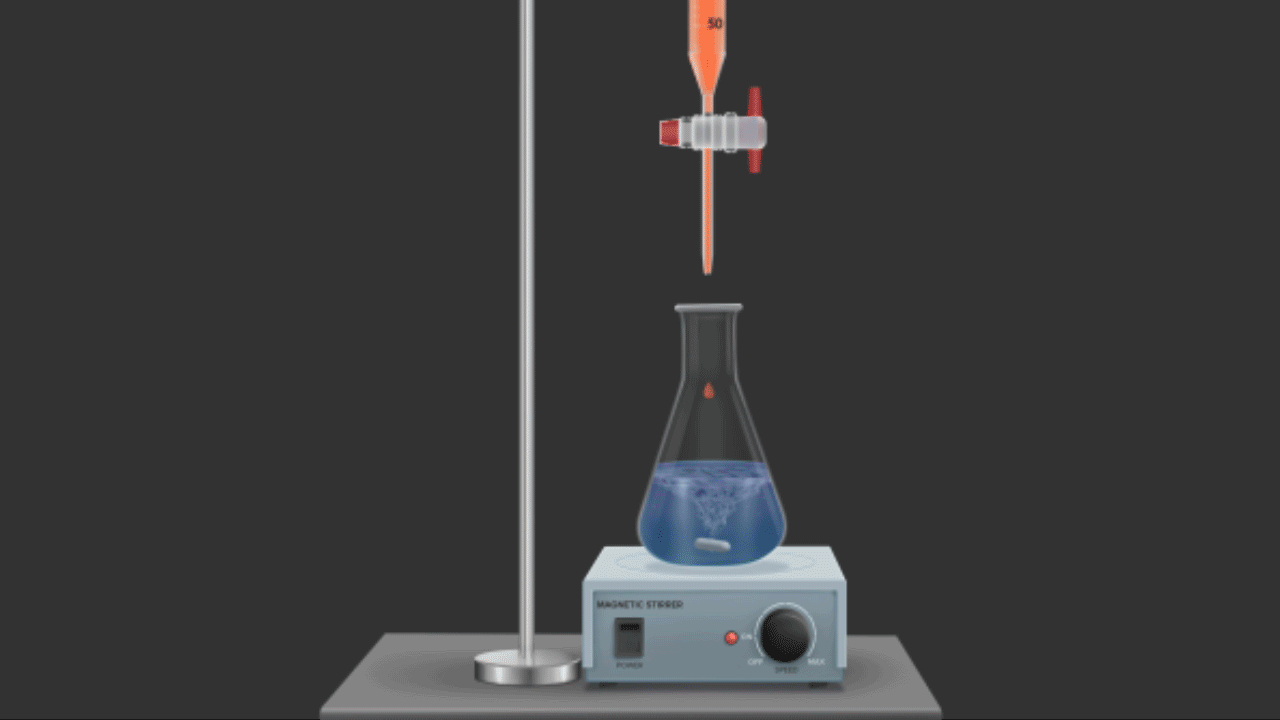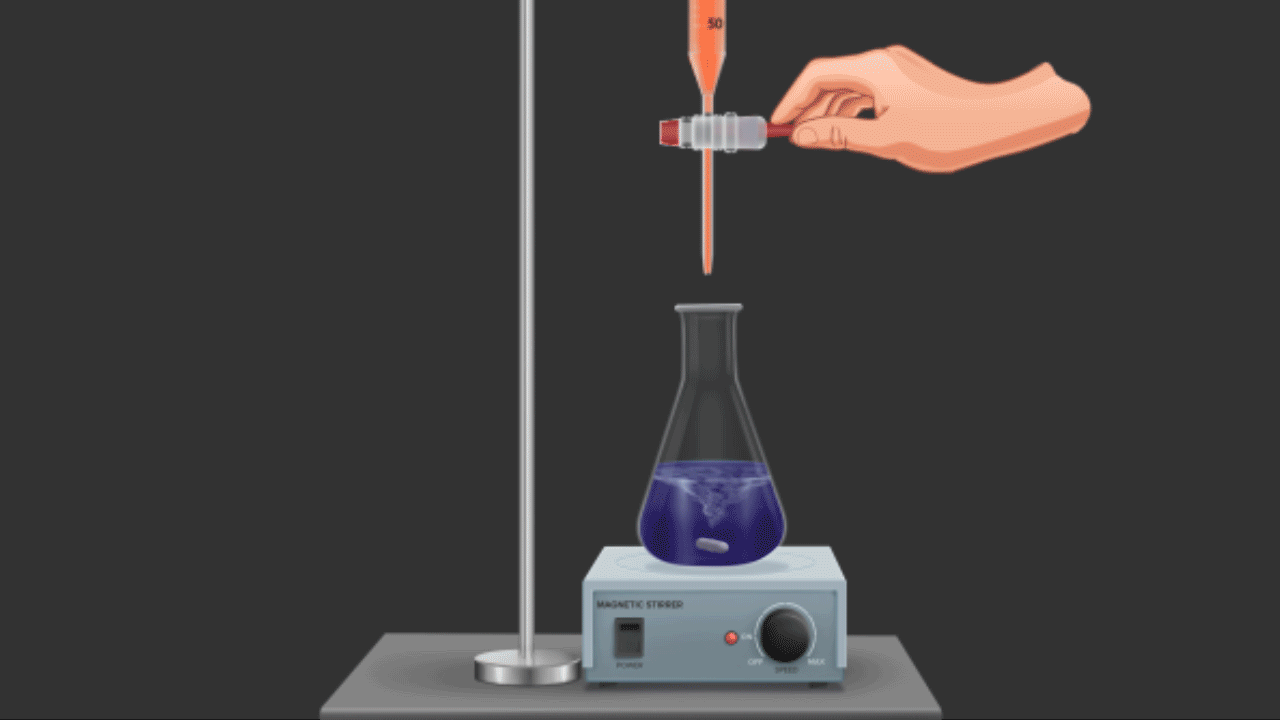
Titration of Cr2O72- against Fe2+ions
Reduction Half-Reaction:
Oxidation Half-Reaction: ……………6
Overall reaction:
Recommended Videos
Titration using acidified KMnO4 and acidified K2Cr2O7 | Chemistry | NEET | Concept of the Day
Practice Problems
1. Find the n-factor of Cr in Cr2O72-(aq) and Cr3+(aq) respectively in the reaction
- 6, 2
- 3, 6
- 2, 6
- 6, 3
Answer: D
Solution:
Let the oxidation state of Cr in Cr2O72- be x.
2x+7(-2)=-2
2x-14=-2
2x=+12
x= +6
Let the oxidation state of Cr in Cr3+ be y.
y=+3
n-factor of Cr in Cr2O72- = 2 |3 - 6| = 6
n-factor of Cr in Cr3+ = |6 - 3| = 3
So, option D is the correct answer.
2. Find the n-factor of C in C2O42- in the reaction
- 2
- 3
- 5
- 1
Answer: A
Solution:
Let the oxidation state of C in C2O42- be x.
2x+4(-2)=-2
2x-8=-2
2x=+6
x= +3
Let the oxidation state of C in CO2 be y.
y+2(-2)= 0
y =+4
n-factor of C in C2O42- = 2 |4 - 3| = 2
So, option A is the correct answer.
3. Pick out the correct statement about redox titrations.
- In permanganate titration, potassium permanganate itself acts as an indicator.
- In permanganate titration, external indicators like K4[Fe(CN)6], are frequently used.
- In dichromate titration, potassium dichromate itself acts as an indicator.
- All are correct statements
Answer: A
Solution: Since KMnO4 is already an intense purple-coloured substance, no indicator is used in these titrations. KMnO4 serves as a self-indicator and loses colour at the endpoint (once it is all used up) when titrated with a reducing agent. A single drop of potassium permanganate added after the reducing agent gives the solution a touch of pink colour.
However, unlike KMnO4, potassium dichromate does not function as a self-indicator because these titrations do not result in a noticeable auto-colour shift. External indicators like potassium ferricyanide, K4[Fe(CN)6], are frequently used in these titrations.
So, option A is the correct answer.
4. In the permanganate redox titrations, potassium permanganate behaves as
- Reducing agent
- Oxidising agent
- Self Indicator
- Both (A) and (C)
Answer: D
Solution: Since KMnO4 is already a highly coloured substance, no indicator is used in these titrations (dark purple). Thus, KMnO4 serves as a self-indicator In redox titrations, acidified potassium permanganate KMnO4 is also employed as an oxidising agent.
If we consider the titration of MnO4- against Fe2+ions,
The oxidation state of Mn decreases from +7 in MnO4- to +2 in Mn2+. If the oxidation state decreases, reduction occurs and hence MnO4- acts as an oxidising agent.
So, option D is the correct answer.
Frequently Asked Questions – FAQ
1. Are there any industrial uses of redox titrations?
Answer: Evaluating the chlorination of public water sources is one of the most significant industrial applications of redox titrations. It employs a method for calculating the amount of residual chlorine by reducing iodide to triiodide ion. It indicates that iodine titration, a process related to redox reactions, is used to disinfect water.
2. Why is potassium dichromate used to determine a reagent in redox titration instead of sodium dichromate?
Answer: In quantitative analysis, a standard solution is a solution with a known strength that is used to estimate the strength of an unknown solution. K2Cr2O7 is preferred over Na2Cr2O7 since the latter is hygroscopic by nature (because Na+ ions are smaller than K+ ions and consequently have a greater propensity for hydration), making it difficult to determine an accurate weight for the creation of a standard solution. When using Na2Cr2O7 as the reference solution for the volumetric analysis, this could lead to inaccurate results.
3. Can HCl act as an acidifying agent in a permanganate-based redox titration?
Answer: Since HCl interacts with permanganate ions to produce chlorine gas, it is not employed in permanganate titrations. Hydrochloric acid is not preferred over sulphuric acid in order to prevent this reaction.
4. How may redox titration be used to control the peroxide content of teeth-whitening products?
Answer: As we know, the oxidation-reduction reaction concept is applied in redox titration. Here, peroxide contained in teeth-whitening products is reduced by various oxidising agents, such as acidified KMnO4 (Potassium permanganate), acidified K2Cr2O7(Potassium dichromate) and iodine solutions. Hence, we can evaluate the amount of peroxide.


