-
Call Now
1800-102-2727
Osazone Formation – Definition, Formation, Tests of Osazone, Practice Problems and FAQ
Meet Madam Moira! Playing with her magical crystal balls, that change their colours and shapes quickly enough when our magic queen uses different ingredients on them! This is her dreamland of magic!
But what if such magic could be turned into reality? You might think that’s incredible. But I would rather say that it is incredibly true! At least in chemistry, this is quite closely feasible!

In chemical analysis of certain carbohydrates (specifically reducing sugars), this can be conceptualised. Osazone is one such magically intriguing compound that is formed by reducing sugars in particular, when reacted by certain ingredients (we are about to learn that in detail here!). Osazones are vividly coloured, crystals that can take multiple-forms and shapes depending on its initiating reactants and are hence highly distinguishable.
In nature, there are many different chemicals and compounds. Each one's characteristics and uses are particular. The osazone test is one such crucial test used in organic chemistry. which is used to distinguish between reducing and non-reducing sugars.
Let's dive in straight!
TABLE OF CONTENTS
- Osazone
- Osazone Formation
- Osazone Test
- Practice Problems
- Frequently Asked Questions – FAQ
Osazone
In organic chemistry, osazones are a carbohydrate derivative generated when reducing sugars react with excess phenylhydrazine at high temperatures.
Osazone Formation
1,2-biarylhyrazone is formed by the reaction of an aldose or ketose with three molar equivalents of aryl hydrazine (like phenylhydrazine). The most common osazone is phenylosazone, formed by the reaction with phenylhydrazine or 2,4-dinitrophenylhydrazine.
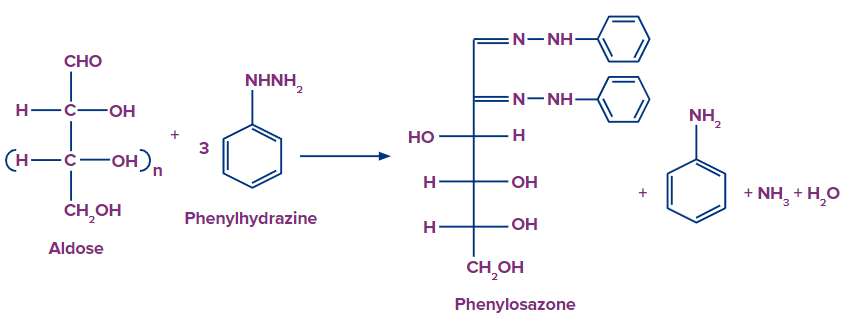
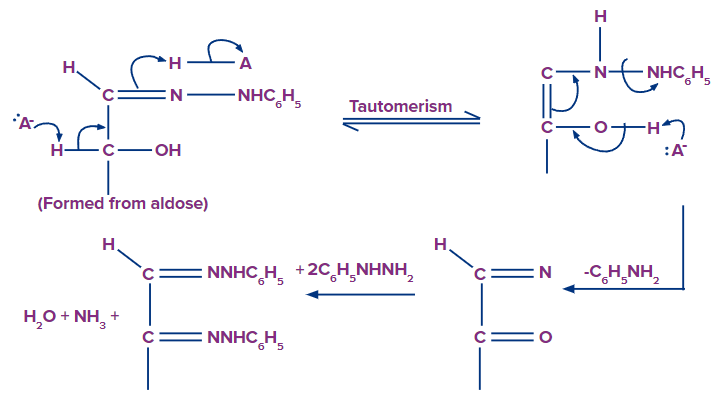
Mechanism of Formation of Phenylhydrazone
Osazone Test
A chemical test for detecting reducing sugars is the osazone test. This test can even distinguish between the reducing sugars on the basis of how fast a complex can be formed. Because of the reagent employed, this test is also known as the phenylhydrazine test.
Objectives
- To identify sugars that are reducing in nature.
- To distinguish between reducing and non-reducing sugars.
- To discriminate between different types of reducing sugars.
Principle
The osazone test is based on a simple principle. This test uses phenylhydrazine in acetate buffer as the reagent. The basis of the osazone test is that phenylhydrazine reacts with the carbonyl group (which should be free) of the carbohydrate to form osazone.
Given below is the reaction showing glucosazone formation when D-glucose interacts with phenylhydrazine.
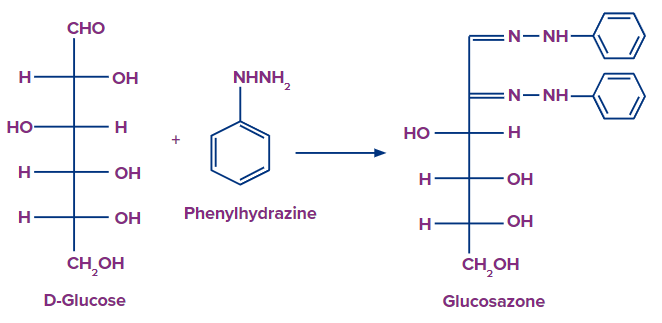
Osazone crystals are yellow-coloured and have a certain solubility, shape, melting point and formation time. Various sugars require different amounts of osazone.
C-2 epimers yield the same osazone as C-1 epimers because both carbon 1 and carbon 2 are engaged in the reaction.
Ketones having the same structure as aldoses below C-2 produce the same osazones, such as fructose and glucose.
Procedure
- 5 mL of the solution under test should be placed in a dry, clean test tube.
- Add glacial acetic acid and the osazone mixture (0.3 g) to the test tube (5 drops).
- Mix everything up thoroughly, and if required, gently heat the test tube in a water bath to help the components dissolve.
- Watch the crystals form at various times while keeping the test tube submerged in the hot water bath.
- Use a modest magnification in a microscope to look at the crystal's shape.
Results and Interpretation
The structure, form, and appearance time of the crystals can be used to distinguish between various sugars.
The following chart shows how to identify reducing sugar using this test.
|
Carbohydrate |
Crystals Appearance time (min) |
Structure of Crystals |
|
Lactose |
30-45 |
Cotton ball/ powder puff shape |
|
Maltose |
30-45 |
Sunflower/ star shape |
|
Galactose |
20 |
Thorny ball shape |
|
Glucose |
5 |
Needle shape |
|
Fructose |
2 |
Needle shape |
With the use of this table, distinct sugars can be identified based on the crystal structure and the timing of manifestation.
Applications
- The osazone test is the sole means to distinguish between lactose and maltose when identifying unknown sugars.
- This is a straightforward test for detecting and differentiating the many sugars used in clinical practice that is also less expensive and time-consuming.
- This test can also detect sugars in plant tissues.
Limitations
Despite being a useful test it has some limitations too.
- Though sucrose is a non-reducing sugar, when it is boiled for longer than 30 minutes, this test yields a positive result for it.
- This test will not be reliable if the sample being tested contains a blend of sugars.
- Additionally, a lot of sugar is necessary for a successful conclusion.
Practice Problems
1. Osazones are generated when reducing sugars are reacted with an excess of
a. Phenylhydrazine
b. Aldose
c. Ketose
d. Alkyl halides
Answer: A
Solution: Osazones are a carbohydrate derivative generated when reducing sugars react with excess phenylhydrazine at high temperatures.
So, option A is the correct answer.
2. What is the purpose of the osazone test?
a. To identify sugars that are decreasing.
b. To distinguish between reducing and non-reducing sugars.
c. To discriminate between different types of reducing sugars.
d. All of the above
Answer: D
Solution: The objective of the osazone test is
- To identify sugars that are reducing in nature.
- To distinguish between reducing and non-reducing sugars.
- To discriminate between different types of reducing sugars.
So, option D is the correct answer.
3. What will be the crystalline structure of fructose after the osazone test?
a. Needle shape
b. Thorny ball shape
c. Sunflower/star shape
d. None of the above
Answer: A
Solution: Different sugars can be identified based on the shape and structure of the crystals as well as their appearance time. The crystalline structure of fructose after the osazone test will be needle-like.
The following chart shows how to identify reducing sugar using the osazone test.
|
Carbohydrate |
Crystals Appearance time (min) |
Structure of Crystals |
|
Fructose |
2 |
Needle shape |
|
Glucose |
5 |
Needle shape |
|
Galactose |
20 |
Thorny ball shape |
|
Maltose |
30-45 |
Sunflower/ star shape |
|
Lactose |
30-45 |
Cotton ball/ powder puff shape |
So, option A is the correct answer.
4. What will be formed when D-glucose interacts with phenylhydrazine?
a. Glucosazone
b. Phenylosazone
c. Aldehyde
d. Ketone
Answer: A
Solution: Glucosazone is formed when D-glucose interacts with phenylhydrazine as shown below.
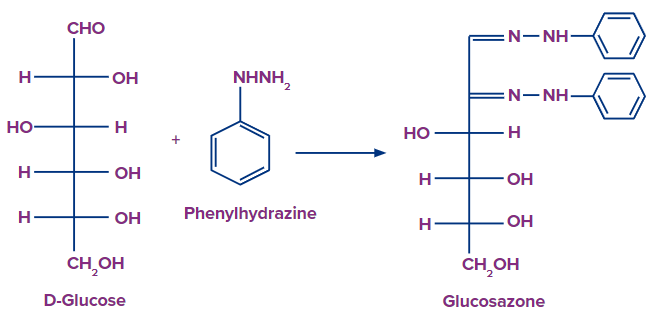
So, option A is the correct answer.
Frequently Asked Questions – FAQ
1. How many carbon atoms are present in glucose?
Answer: We know that the molecular formula of Glucose is C6H12O6, so it has 6 carbon atoms.
2. Is glucose a reducing sugar?
Answer: Glucose is a reducing sugar because it is an aldose, which means it has an aldehyde group in its open-chain form. In general, carboxylic acids are quickly oxidised from aldehydes.
3. What is the purpose of phenylhydrazine?
Answer: Phenylhydrazine is a yellow-to-brown crystal or a yellowish oily liquid that can be found in a variety of forms. This molecule is utilized as a chemical intermediary primarily in the pharmaceutical, agrochemical, and chemical industries around the world.
4. What are reducing sugars?
Answer: Any carbohydrate capable of being oxidised and causing the reduction of other compounds without needing to be hydrolyzed first is referred to as reducing sugar. Reducing sugars are carbohydrates that, due to the presence of free aldehyde or ketone groups, can act as reducing agents. Sugar reduction has a sweet flavour.
5. What is the difference between blood sugar and glucose?
Answer: The main distinction between blood sugar and glucose is that blood sugar refers to glucose that is transported by the bloodstream to all of the body's cells, whereas glucose is a simple sugar and the most abundant monosaccharide.


