-
Call Now
1800-102-2727
Reactions of Haloarenes: Nucleophilic Substitution Reactions, Reaction with Metals, Practice Problems and Frequently Asked Questions(FAQs)
Can you believe that haloarenes can be produced by marine organisms?
Yes it’s true.

Haloarenes can be produced by marine organisms that can use chloride and bromide found in ocean waters. Haloarenes are abundant in nature and have been shown to have a variety of medicinal properties. As a result, various haloarenes reactions occur both artificially and naturally. Many other chlorinated and brominated aromatic compounds exist in nature, including , and numerous pyrrole derivatives.
Table of content:
- Nucleophilic substitution reactions
- Reaction with metals
- Practice problems
- Frequently asked questions(FAQs)
Nucleophilic substitution reactions:
In this type of reaction, a nucleophile (nucleus-seeking species) reacts with the substrate (the haloarene). The haloarene substrate carries a partial positive charge on the carbon atom bonded to halogen. The nucleophile replaces the halogen atom that leaves as the halide ion. The halogen atom is known as the leaving group. This substitution reaction is called the nucleophilic substitution reaction because it is initiated by a nucleophile.
Haloarenes do not undergo nucleophilic substitution reactions readily. They do, however, undergo these reactions under certain specific reaction conditions.
The following are the key reasons for haloarenes decreased reactivity to nucleophilic substitution reactions:
(i) Resonance:
The electrons present in the benzene ring will be in conjugation with the halogen atom in haloarenes. This causes resonance between the benzene ring and the halogen atom, giving the C-X bond a partial double bond nature.
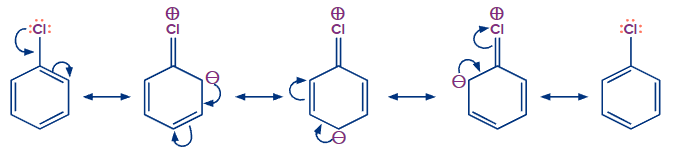
As a result, cleaving the C-X bond is more difficult compared to haloalkanes (alkyl halides). As a result, the C-X bond in haloarenes is difficult to rupture by a nucleophile, making them less reactive to nucleophilic substitution reactions.
(ii) Difference in Hybridisation of Carbon Atom in C-X Bond:
The halogen group is attached to a sp2 hybridized carbon atom of the benzene ring in haloarenes. In haloalkanes, however, the halogen atom is attached to the sp3 hybridized carbon atom.
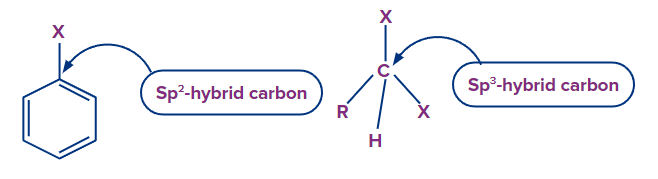
As a result, the % S character in haloarenes sp2 carbon is stronger than in haloalkanes sp3 carbon. As a result, the electronegativity of the sp2 carbon atom is greater than that of the sp3 carbon atom.
Thus, the haloarenes sp2 hybridized carbon atom has a greater electron-withdrawing capacity and can hold the electron pair of the C-X bond more tightly than the haloalkanes sp3 hybridized carbon. As a result, the bond length in haloarenes is shorter than in haloalkanes. A stronger bond results from a shorter bond length. For example, the C-Cl bond length in haloalkane is 177 pm whereas in haloarene it is 169 pm.
Because it is more difficult to break a shorter bond than a longer bond, haloarenes are less reactive to nucleophilic substitution reactions than haloalkanes.
(iii) Phenyl Cation Instability:
In haloarenes, cleavage of the C-X bond results in the formation of phenyl cation and a halide ion X-. Because of the presence of a positive charge on the electronegative sp2 C-atom, the resulting phenyl cation is highly unstable. Furthermore, resonance cannot be used to stabilize the phenyl cation. As a result, the reaction mechanism has been ruled out.
As a result, the instability of phenyl cation reduces the haloarene's reactivity to nucleophilic substitution reactions.
(iv) Electronic Repulsion:
Haloarenes are made up of a large electron-rich aryl group. When an electron-rich nucleophile approaches the electron-rich phenyl ring (arenes) in haloarenes, electronic repulsion may occur.
As a result, the haloarene's reactivity to nucleophilic substitution reactions decreases.
Conditions when Haloarenes are Reactive:
We know that haloarenes are generally unreactive or only slightly reactive in nucleophilic substitution reactions. However, haloarenes can be reactive to nucleophilic substitution reactions in some cases. The following example will help you understand how certain haloarenes react to nucleophilic substitution reactions under extreme conditions.
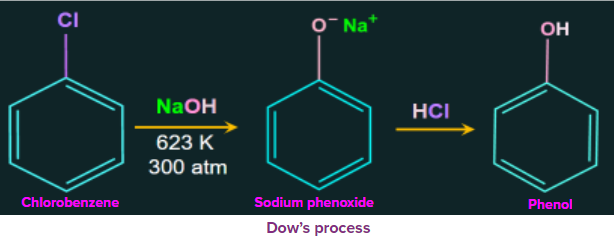
Replacement by Hydroxyl Members:
The reactant chlorobenzene undergoes nucleophilic substitution when heated with aqueous sodium hydroxide at temperatures of 623 K and 300 atm to get sodium phenoxide ion. The sodium phenoxide ion is then treated with dilute HCl, yielding the end product, phenol. This is known as Dow's method.
The presence of an electron-withdrawing group, on the other hand, increases the reactivity of haloarenes to nucleophilic substitution reactions. If an electron-drawing group, such as -NO2, is present in the ortho, para, or both ortho and para positions. Haloarenes reactivity to nucleophilic substitution increases, and the reaction requires much less drastic conditions to proceed.
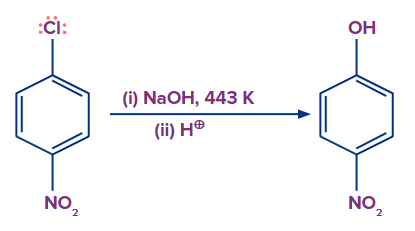
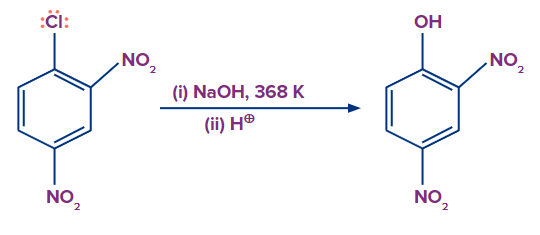
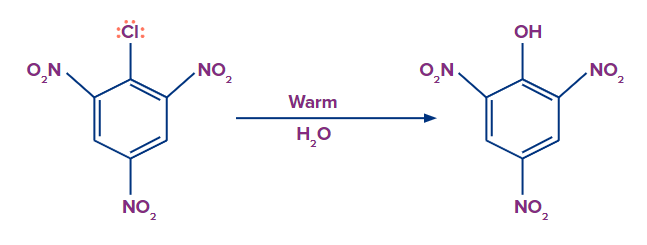
Mechanism:
A nucleophile approaches and attacks the C-X bond, and the electrons move in such a way that the electron density in the benzene ring delocalised at the Ortho and Para positions. If an electron withdrawing group is present at the Ortho and Para positions of the benzene ring in such a case, it will stabilize the negative charge on the carbon atom through withdrawal.
As a result, the presence of electron withdrawing groups such as -NO2 in the ortho and para positions facilitates the nucleophile's attack. Furthermore, carbanion stabilization occurs via resonance as well as electron withdrawing groups such as -NO2.

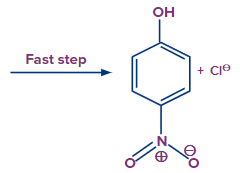
The reaction will proceed in a series of fast and slow steps, resulting in the formation of a high resonance stabilized sigma complex. Finally, the -electrons will be restored when the negative charge delocalized by breaking the Cl bond. Finally, the product will be formed.
Reaction with metals:
Haloarenes undergo few reactions with metals. Two primary reactions are:
- Wurtz-Fittig Reaction
- Fittig Reaction
- Grignard reagent
1. Wurtz-Fittig reaction:
When an alkyl halide and an aryl halide react with sodium in the presence of dry ether and sodium, an alkyl arene is formed.

2. Fittig reaction:
When a mixture of haloarenes reacts with sodium in the presence of dry ether, a diphenyl (Diarene) is formed.

3. Grignard reagent:
Aryl halides on reaction with magnesium metal in the presence of ether solvent gives grignard reagent.

Practice problems:
Q.1. Because of the large difference in their structures, the ortho and para isomers of dichlorobenzene formed by electrophilic substitution of arenes can be easily separated by:
(A) Melting points (B) Boiling points
(C) Solubilities (D) Densities
Answer: (A)
Solution: The main criterion for determining melting point order is molecular packing. p-Dichlorobenzene is symmetrical and fits easily into a crystal lattice. As a result, intermolecular forces of attraction are stronger than in the ortho and meta isomers. Because the ortho and meta isomers structures are not as symmetrical and do not form a close lattice structure, the intermolecular forces of attraction are comparatively weaker. Because the para isomer has strong intermolecular forces of attraction, it takes more energy to melt and thus has a higher melting point than the ortho and meta isomers.
Q.2. What is the catalyst in the reaction of a primary alcohol with HCl to obtain chloroalkane?
(A) Anhydrous ZnCl2 (B) Conc. H2SO4
(C) Red phosphorous (D) Pyridine
Answer: (A)
Solution: The presence of anhydrous ZnCl2 is to break the C-O bond in alcohols. Anhydrous ZnCl2 is a Lewis acid and reacts with the oxygen of the alcohol group.
Q.3. Which of the following cannot act as a catalyst in electrophilic substitution reactions of arenes?
(A) FeCl3 (B) FeBr3
(C) NH3 (D) AlCl3
Answer: (C)
Solution: The presence of a Lewis acid catalyst that acts as a halogen carrier is required for the electrophilic substitution reactions of arenes. NH3 is a lewis base which cannot act as a catalyst in electrophilic substitution reactions of arenes.
Q.4. Which of the following dichloro arenes is formed when excess chlorine is used in the electrophilic substitution of toluene?
(A) ortho and meta (B) ortho and para
(C) meta and para (D) ortho, meta and para
Answer: (B)
Solution: When excess halogen is present, the second halogen is also incorporated into the aromatic ring at ortho and para positions relative to the first halogen. This is due to the ortho and para directing nature of the -CH3 group in toluene.
Frequently asked questions(FAQs):
Q1. How electron withdrawing groups at meta positions affect the nucleophilic substitution reactions?
Answer: However, the presence of the electron-withdrawing group in meta-position has no significant effect on the reactivity of haloarenes.
In the case of meta-nitrobenzene, none of the resonating structures has a negative charge on the carbon atom with the -NO2 group. As a result, the presence of a nitro group in meta-position has no effect on the reactivity of haloarenes to the nucleophilic substitution reaction. It does not stabilize the negative charge in the same way that ortho and para positions do.
Q2. Why are haloarenes insoluble in water but are soluble in benzene?
Answer: Haloarenes are insoluble in water because they cannot form H-bonds with water and cannot break existing H-bonds in water.
However, in accordance with the general principle of solubility, i.e., like dissolves like, haloarenes are soluble in hydrocarbon solvents such as benzene, petroleum ether, and so on due to the presence of a large hydrocarbon part (benzene ring).
Q3. Why can't aryl halide be made by combining phenol with HCl in the presence of ZnCl2?
Answer: In the case of alkyl halide, carbocation formation occurs, which eventually reacts with HCl to form an alkyl halide.
However, in order for aryl halide to react with HCl in the presence of ZnCl2, phenyl carbocation formation must occur, which is not possible because it is a highly unstable structure that never exists in free state. As a result, no aryl halide is formed.
Q4. What causes haloalkanes to be more reactive than haloarenes?
Answer: Because halogens have a lone pair of electrons, the C-X bond (carbon halogen bond) in haloarenes has a double bond nature. The C-X bond in alkyl halides is weaker in comparison to the resonance stabilized C-X bond in aryl halides. As a result, haloalkanes react more quickly than haloarenes.


