-
Call Now
1800-102-2727
Nucleophilic Substitution Reaction-SNi, SNNGP - Mechanism and Example of SNi , Characteristics, Conditions, Mechanism, Example of SNNGP
Let's consider a situation. A family is shifting to a new place, and they are carrying their goods to the new home. Their neighbours see them, and they say that they are coming downstairs. They introduce themselves to each other. They all are shifting the packages. The new family is very thankful to them and it is observed that all of the packages have been efficiently placed with the help of neighbours, the time is reduced, and the rate has been increased. Similarly, in NGP(neighbouring group participation), the neighbouring nucleophile attacks and helps in the removal of the leaving group and increases the rate of the reaction.

TABLE OF CONTENTS
- Introduction
- Mechanism of SNi Reaction
- Example of SNi
- SNNGP Reaction
- Characteristics of SNNGPReaction
- Conditions for SNNGP
- Mechanism of SNNGP Reaction
- Example of SNNGP
- Practice Problems
- Frequently Asked Questions - FAQs
Introduction
In SNi , S stands for substitution, N stands for nucleophilic, i stands for internal. In the SNi (internal nucleophilic substitution), a part of the leaving group must be able to attack the substrate detaching itself from the rest of the leaving group in the process. In the SNi reaction, the mechanism proceeds with the retention of configuration.
Example- Reaction of alcohol with SOCl2 Thionyl chloride (SOCl2) converts primary and secondary alcohols to alkyl chlorides with the retention of configuration.

Mechanism of SNi Reaction
Step 1: Reaction of alcohol with SOCl2
Alcohol reacts with thionyl chloride resulting in the formation of alkylchlorosulphite.

Step 2: Dissociation of alkylchlorosulphite
Second step is the same as the very first step of the SN1 mechanism i.e., dissociation into an intimate ion pair.

Step 3: Attack of leaving group
Part of the leaving group attacks, necessarily from the front since it is unable to get to the rear side. It results in retention of configuration.
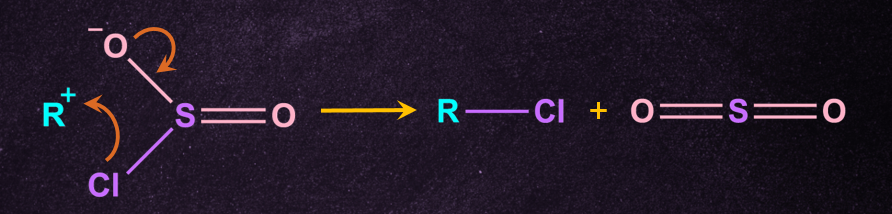
If the same reaction occurs in the presence of pyridine, an inverted product is formed. Pyridine (C5H5N) is often included to promote the reaction.

The mechanism involved in the above reaction is followed:
1. Reaction of alcohol with SOCl2
Alcohol reacts with thionyl chloride resulting in the formation of an alkylchlorosulphite.

2. Formation of pyridinium- alkylsulphite intermediate
The alkylchlorosulphite intermediate formed then reacts rapidly with another molecule of pyridine, to give a new intermediate called pyridinium alkyl sulphite.
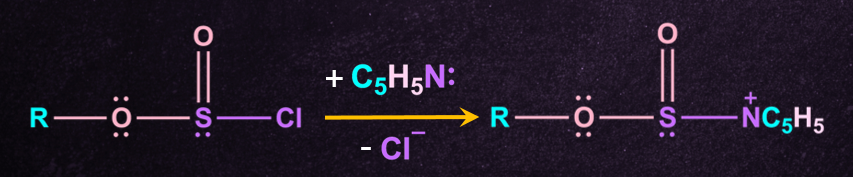
3. Attack by chloride anion
A chloride anion then attacks the substrate carbon, displacing the sulphite leaving group.
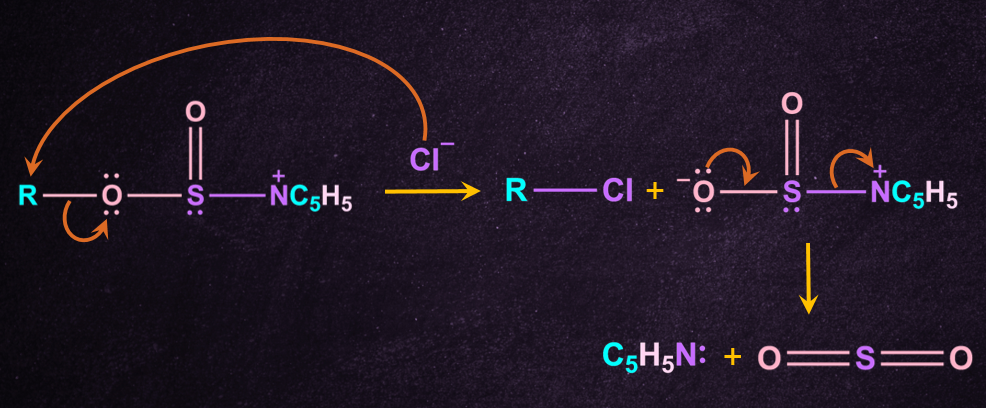
Inversion of the configuration takes place because free Cl- attack from the opposite side of the leaving group to give inverted R-Cl and further cleaves to give pyridine(NC5H5 ) and SO2.
Example of SNi Reaction:
Reaction of Alcohol with PCl5

Reaction with PCl5 follows the same steps of mechanism as that of SOCl2 is followed.
SNNGP Reaction
In SNNGP, S stands for substitution, N stands for nucleophilic, NGP stands for neighbouring group participation. It is also known as anchimeric assistance, which in Greek, it means “Adjacent part”.
Note: Rate of intramolecular reaction( i.e.when two reacting groups in the same molecule) is faster than intermolecular( i.e. when two reacting groups in separate individual molecules).
Characteristics of SNNGP Reaction
1. The rate of reaction is greater than expected.
2. The configuration at a chiral carbon is retained such that the configuration at a chiral carbon is not inverted or racemised.
Conditions for SNNGP Reaction
Nucleophile should be present within the molecule/ internally. Generally, the nucleophile and leaving group should be present anti to each other at 1,2 position. The concentration of nucleophile (external) should be less.
Mechanism of SNNGP Reaction
Reactions, in which there is usually a group with an unshared pair of electrons, present at β-position to the leaving group (or sometimes farther away). The mechanism operating in such cases is called the neighbouring-group participation mechanism. It consists essentially of two SN2 substitutions. Each causing an inversion so the net result is retention of configuration.
Step 1: Intramolecular nucleophilic substitution reaction
The neighbouring group acts as a nucleophile, pushing out the leaving group but still retaining attachment to the molecule.

Step 2: External nucleophilic substitution reaction
The external nucleophile then displaces the neighbouring group by attacking from the backside.
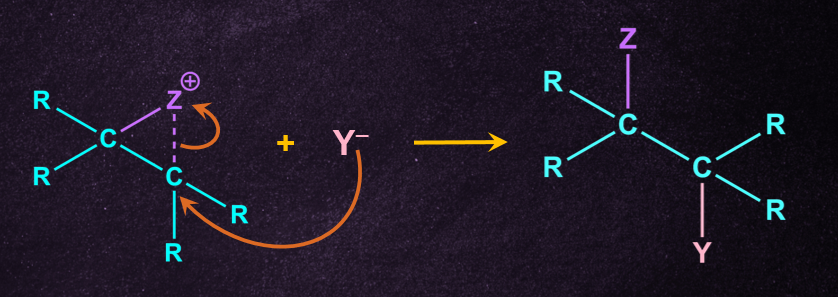
Below given is the table of groups behaving as Neighboring Groups(internal nucleophile).
|
Sulphur |
|
|
Carboxylic acid derivatives |
-COOˉ, -COOR, -OCOR |
|
Oxygen |
-OR, -OH, -Oˉ |
|
Nitrogen |
-NH2, -NHR, -NR2 |
|
Halides |
-I, -Br, -Cl |
Examples of SNNGP Reaction
Neighbouring Group Participation by:
1. Involving species having lone pair

2. Aromatic rings as neighbouring groups

3. Cyclopropyl methyl system
Cyclopropyl methyl substrates solvolyze with abnormally high rates. The products often include not only cyclopropyl-methyl, but also cyclobutyl and homoallylic compounds.
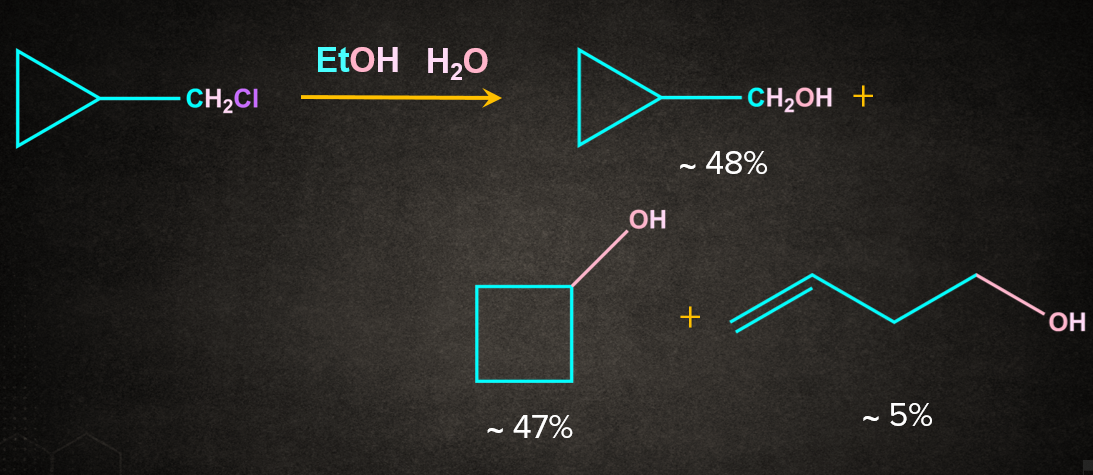
Practice problems
- Major product of the following reaction is:

A). 
B). 
C). 
D). None of the above
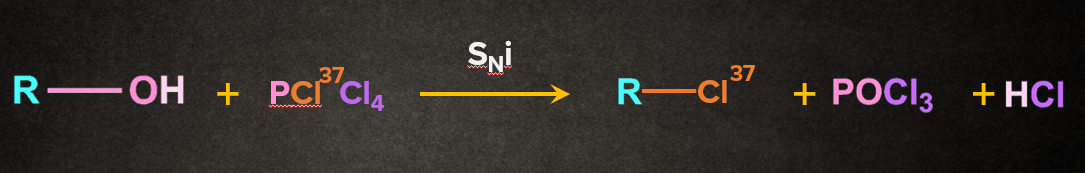
Answer: B
Solution: The above reaction proceeds via SNi mechanism.
2. Reaction of alcohol with SOCl2 in the presence of pyridine occurs via _______ of configuration.
A). Inversion
B). Retention
C). Both of the above
D). None of the above
Answer: A
Solution: Reaction of alcohol with SOCl2 in presence of pyridine, gives the inverted product. Pyridine
(C5H5N) is often included to promote the reaction.
3. Write the product of the following reaction with proper stereochemistry.
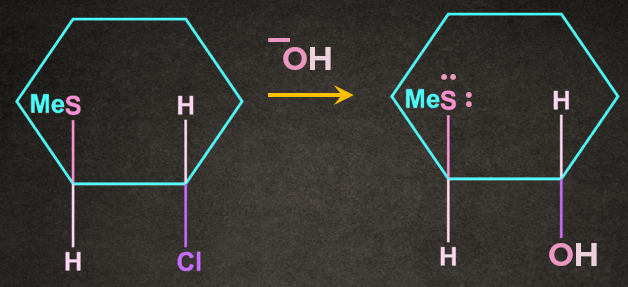
A). 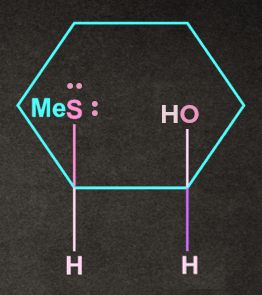
B). 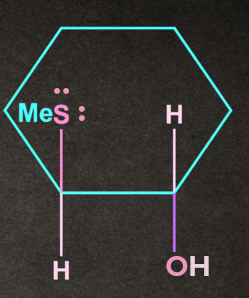
C). Both of the above
D). None of the above
Answer: B
Solution: 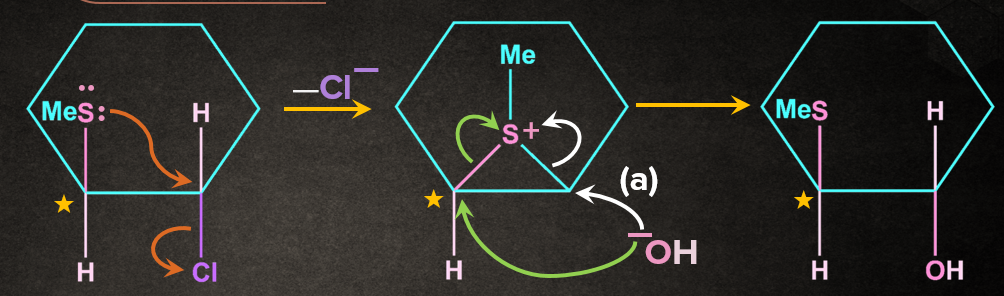
4. Write the product of the following reaction with proper stereochemistry.
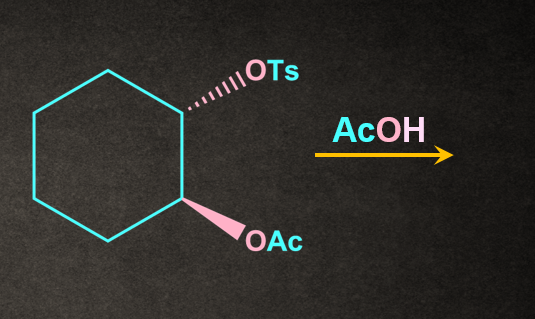
A). 
B). 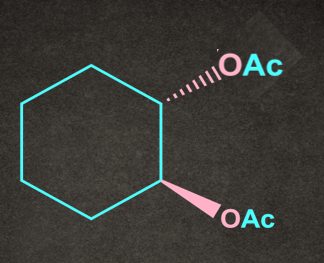
C). Both of the above
D). None of the above
Answer: B
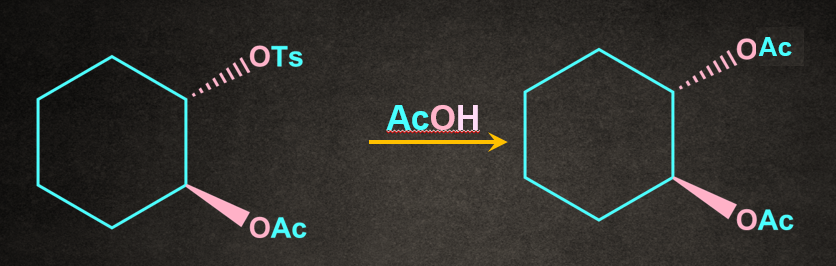
Solution: The given reaction proceeds via SNNGP mechanism. Therefore, retention of the configuration takes place.
Frequently Asked Questions-FAQs
Q1. What is a nucleophilic substitution reaction?
Answer: The replacement of an atom or group by any other atom or group in a molecule is known as a substitution reaction. If a substitution reaction is brought about by a nucleophile, then it is known as a nucleophilic substitution reaction.
Q2. What is a leaving group?
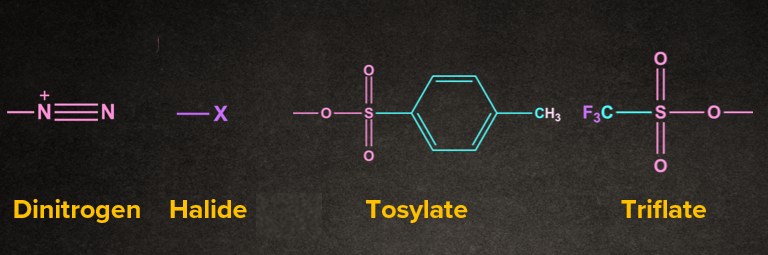
Answer: The part of the reactant molecule which gets cleaved is called a leaving group. Generally poor bases are a good leaving group.
1. Nucleofuge: Leaving group that carries away an electron pair or negative charge. For nucleofuge, weaker bases are good leaving groups.
2. Electrofuge: Leaving group that comes away without an electron pair or having positive charge.
Generally, the leaving group carries away an electron pair.
Example:
Q3. What is an intimate ion pair?
Answer: When a polar molecule dissociates into a cation and an anion in a solvent, they can stick together for a short period, producing an intimate ion pair, before diffusing away.
Q4. What is a racemic mixture?
Answer: Racemic mixture is the equimolar mixture of d and l forms of an optically active compound (enantiomers).


