-
Call Now
1800-102-2727
Nitrogen Dioxide – Introduction, Source, Structure, Preparation, Properties, Uses, Practice problems and FAQ
Our five fingers are not all the same height or utility. They have distinct shapes and abilities. Similarly, there are differences in properties, structures, and other characteristics among the various nitrogen oxides. Nitrogen dioxide is one of these "not so ideal" offspring of nitrogen and oxygen. He is extremely hazardous to the environment!
Volcanoes, floods, biological collapse, and lightning are some examples of natural causes of nitrogen dioxide emission. Nearly 24 million tonnes of nitrogen oxides are added to the atmosphere each year by human activity. Vehicles, power plants, industrial pollutants, and off-road sources like gardening, lawn, and construction equipment all contribute to nitrogen dioxide emissions. Fossil fuels are burned by all of these sources. High exposures can occur for people who live or work close to busy roads.
But how does it actually affect us and what are its major properties? How do we even prepare it?
Let’s find out!
TABLE OF CONTENTS
- Nitrogen Dioxide - Introduction and Source
- Nitrogen Dioxide - Structure
- Nitrogen Dioxide - Preparation
- Nitrogen Dioxide - Physical Properties
- Nitrogen Dioxide - Chemical Properties
- Nitrogen Dioxide - Uses
- Nitrogen Dioxide - Health hazards
- Practice Problems
- Frequently Asked Questions - FAQ
Nitrogen Dioxide - Introduction and Source
Nitrogen dioxide (NO2) is a very important oxide of nitrogen. It is a highly reactive and extremely toxic gas. It is known as nitrogen (IV) oxide. It is one of the most common air contaminants in the atmosphere that absorbs UV radiation and prevents it from reaching the Earth's surface.
Nitrogen (IV) oxide is a yellowish-brown liquid or a reddish-brown gas. Vapours of NO2 are thicker than that of air.
Nitrogen dioxide also serves as a precursor to the creation of nitrate aerosols and nitrosamines, both of which have health implications. Nitrogen oxides are one of the air pollutants for which regulations and frequent restrictions have been created due to the volume produced and the potential for widespread negative impacts on public health and welfare.
Sources of Nitrogen Dioxide
- NO2 enters the environment by natural processes such as stratospheric entry, bacterial respiration, volcanoes, and lightning. NO2 is a trace gas in the Earth's atmosphere, where it absorbs sunlight and regulates the chemistry of the troposphere, particularly in determining ozone concentrations, according to these sources.
- Combustion of fuels from transportation or heavy scale industries, accounts for almost 98% of man-made NO2 emissions, with stationary sources accounting for the bulk. Combustion-generated nitrogen oxides are mostly exhaled as nitric oxide, NO, a comparatively innocuous gas that is quickly transformed to the lethal nitrogen dioxide in the atmosphere. Nitrogen dioxide has a negative impact on human respiratory functioning, and prolonged exposure can lead to a rise in respiratory illnesses.
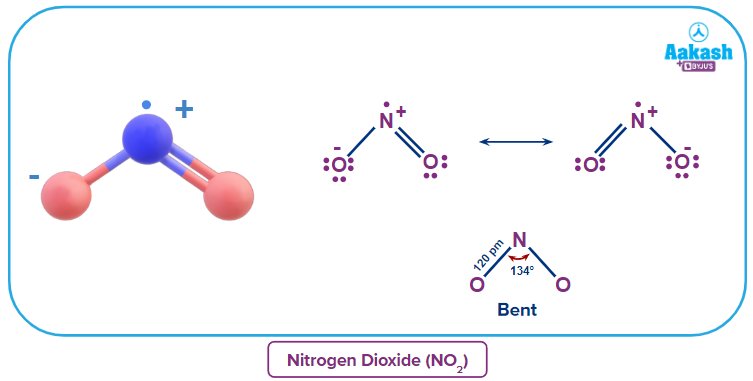
Nitrogen Dioxide - Structure
The covalent nitrogen dioxide molecule (NO2) has a core nitrogen atom that is single-bonded to an oxygen atom and double-bonded to another oxygen atom. The structure of NO2 is bent and is also known to undergo resonance in the following way.
Nitrogen Dioxide - Preparation
- Some metal nitrates undergo heat degradation (thermal decomposition) to produce nitrogen dioxide.
![]()
- Nitric oxide in the presence of oxygen quickly forms nitrogen dioxide.
- Oxidation of metals by concentrated nitric acid produces nitrogen dioxide.
- In the laboratory, NO2 may be made via a two-step process in which nitric acid is dehydrated to generate dinitrogen pentoxide, which is then thermally decomposed.
- Standing nitric acid decomposes into brown nitrogen dioxide. This is why, despite the fact that fresh nitric acid is colourless, it becomes dark with time.
- On adding concentrated nitric acid to tin, nitrogen dioxide along with metastannic acid is obtained.
Nitrogen Dioxide - Physical Properties
- It is a reddish-brown poisonous gas and is very reactive.
- It is an acidic oxide.
- The density of NO2 is 1.880 g dm-3
- Its melting point is −9.3 °C and boiling point is 21.15 °C.
- Its molar mass is 46.006 g mol-1.
- It hydrolyses in water and is also soluble in nitric acid, chloroform and carbon tetrachloride.
- Its refractive index at 20 °C is 1.449 .
Nitrogen Dioxide - Chemical Properties
- Thermal property: At low temperatures, NO2 changes to colourless dinitrogen tetroxide (N2O4), which then reverts back to NO2 at higher temperatures. Hence, NO2 and N2O4 exists in a dynamic equilibrium.
2NO2 ⇌ N2O4
- NO2 is an excellent oxidant due to the fragility of the N–O bond. As a result, many molecules, such as hydrocarbons, may combust, sometimes explosively.
- The weakness of the N-O bond suggests that The N-O bond's frailty suggests that NO2 is a good oxidiser. As a result, it will burn various chemicals, including hydrocarbons, sometimes explosively.
- NO2(N2O4) is a strong oxidising system.
- Upon hydrolysis, nitrogen dioxide produces nitrous acid and nitric acid.
- To make anhydrous metal nitrates from oxides, NO2 is employed.
- Alkyl and metal iodides produce equivalent nitrites in presence of nitrogen dioxide.
Nitrogen Dioxide - Uses
- NO2 is employed as a nitrating agent in the production of chemical explosives, as a nitrating intermediary in the production of nitric acid.
- It is used as a polymerisation inhibitor for acrylates, as a bleaching agent for flour.
- It is used as a room temperature sterilising agent.
- It was utilised in the Titan rockets, the Project Gemini launch, the Space Shuttle's manoeuvring thrusters, and unmanned space probes sent to numerous planets.
- It is also employed as an oxidiser in rocket fuel, such as in red fuming nitric acid.
- It is used to make oxidised cellulose molecules.
- As a catalyst, it is used in several reactions.
- It is an intermediate used in the process of making sulphuric acid.
- As a nitrating agent, it is used in various organic reactions.
Nitrogen Dioxide - Health Hazards
Nitrogen dioxide is an irritant gas that causes inflammation of the lungs at high concentrations. NO2 is mostly harmful to those who have respiratory problems that create a lot of inflammation in their airways. Long-term exposure reduces lung capacity, raises the risk of respiratory problems, and intensifies allergic reactions. NO2 also leads to the creation of fine particles (particulate matter) and ozone at ground level, both of which have negative environmental consequences.
Nitrogen dioxide (NO2) exposure can be lethal at high doses. When it comes into contact with the eyes and skin, it generates a burning feeling. When in liquid form it causes frostbite. It is said to generate methemoglobin when it reacts with blood. When heated to decompose, it releases toxic fumes of nitrogen oxides.
Practice Problems
Q. 1. NO2 is paramagnetic as
A. It has a positive charge
B. It has one electron pair
C. It has one odd-electron
D. It exists as a dimer
Answer: NO2 is paramagnetic due to the presence of one odd electron.
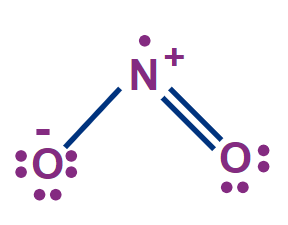
So, option C) is the correct answer.
Q. 2. The oxidation state of nitrogen in NO2 is
A. +1
B. +2
C. -2
D. +4
Answer: Let the oxidation state of N in NO2 be ‘x'
So,
∴ x=+4
So, option D) is the correct answer.
Q. 3. Which of the following factors prevents nitrogen oxide from being directly analysed using UV and visible analysers?
A. Very low range
B. Less Accuracy
C. Contamination of Sample
D. Transparent in UV visible range
Answer: Since nitrogen oxide is transparent in UV visible regions, it cannot be directly analysed with UV and visible analysers. As a result, it is transformed into nitrogen dioxide before being analysed.
So, option D) is the correct answer.
Q. 4. What is the method of conversion of NO to NO2 in a chemiluminescence analyser?
A. Treating sample gas with pressurised oxygen
B. Treating sample gas with ozone
C. Treating sample gas with oxygen at low pressure
D. Treating sample gas with water at high pressure
Answer: By treating sample gas with ozone, NO is transformed to NO2 for examination in a chemiluminescent analyser.
So, option B) is the correct answer.
Frequently Asked Questions - FAQ
Q1. What are the adverse impacts of inhaling nitrogen dioxide?
Answer: Increased risk of respiratory problems is the main effect of breathing at excessive nitrogen dioxide levels. Nitrogen dioxide can lower immunity to lung infections by inflaming the lining of the lungs. Wheezing, coughing, colds, pneumonia, and bronchitis problems might result from this.
Q2. How does NO2 impact the environment?
Answer: The presence of NO2 in the atmosphere aids the development and change of other air pollutants such as ozone and particulate matter, as well as acid rain. Increased amounts of nitrogen dioxide are hazardous to vegetation, causing damage to the foliage, slowing growth, and reducing crop yields.
Nitrogen dioxide has the ability to fade and discolour pieces of furniture and fabrics, as well as reduce visibility and interact with surfaces.
Acid rain is created when NO2 and other NOx in the atmosphere combined with water, oxygen, and other compounds. Sensitive habitats, such lakes and forests, are harmed by acid rain. However, visibility is decreased because the nitrate particles produced by NOx make the air murky and challenging to see (like the pollution in Delhi). Nutrient pollution of coastal waters is a result of NO2 in the atmosphere.
Q3. How are we and our environment exposed to NO2?
Answer: The main cause of NO2 in outdoor urban air is transportation. Automobiles and other combustion processes release nitric oxide (NO), which reacts with oxygen in the air to form nitrogen dioxide (NO2). Unvented heaters and gas stoves are the main sources of NO2 indoors. A range of gaseous air pollutants, including nitrogen dioxide, are created by vehicle traffic and other fossil fuel combustion processes.
Q4. How to control the emission of nitrogen dioxide from vehicles and industries?
Answer: Reburning, combustion staging, gas recirculation, lower air preheat and firing rates, water or steam injection, and low excess air (LEA) firing are a few examples of process improvements in vehicles and industries that can be followed. These changes have the potential to cut NOx emissions by 50 to 80 per cent.


