-
Call Now
1800-102-2727
Mohr’s Salt – Structure, Preparation, Properties, Applications, Practice Problems and FAQ
AA batteries are used in practically every domestic electronic device's remote. The market is filled with several AA battery varieties. Lithium and alkaline batteries are two of the most significant among them.

Do you know which AA battery type has the longest life?
Lithium is a light metal. Compared to alkaline or other batteries of the same size, lithium batteries have a higher charge density and can thus store more energy. Lithium batteries, therefore, have a longer lifespan than other battery kinds.
Why are batteries even a topic of discussion? Does this matter at all for the concept page being discussed here?
It does have a purpose. There is a substance that has a longer shelf life than other species that can serve as a source of ferrous ions (Fe2+), similar to how lithium batteries survive longer than other batteries. Additionally, it resists oxidation when exposed to air. The name of this substance is Mohr's salt.
We will learn more about Mohr's salt, its structure, preparation, properties, and applications on this concept page.
TABLE OF CONTENTS
- Mohr’s Salt
- Mohr’s Salt – Structure
- Mohr’s Salt – Preparation
- Mohr’s Salt – Properties
- Mohr’s Salt – Applications
- Practice Problems
- Frequently Asked Questions – FAQ
Mohr’s Salt
Ammonium iron (II) sulphate, also known as Mohr’s salt is an inorganic salt that has the molecular formula FeSO4.(NH4)2SO4.6H2O. It contains two cations namely ammonium (NH4+) and ferrous (Fe2+). It is a double salt of ferrous sulphate and ammonium sulphate.

Mohr's salt, which quickly crystallises and produces crystals that are remarkably resistant to oxidation in the presence of air, is an essential laboratory reagent.
It should be noted that German chemist Karl Friedrich Mohr inspired the name of Mohr's salt. Mohr's salt, like the majority of other ferrous sulphate salts, dissolves in water to create an aqua complex with the chemical formula [Fe(H2O)6]2+. It should be noted that the molecular geometry of this aqua complex is octahedral. Mohrite is the name for Mohr's salt in its mineral form.
Mohr’s Salt – Structure
It is known that Mohr's salt is a member of the family of double sulphates known as Tutton's salts, also known as Schonites. The crystals of this family, including Mohr's salt, have a monoclinic geometry. [Fe(H2O)6]2+ make up the octahedral centres in the bonding patterns of Mohr's salt. Additionally, it is known that there exist hydrogen bonds between these centres and the ions of ammonium and sulphate.

Mohr’s Salt – Preparation
Ammonium sulphate and hydrated ferrous sulphate are commonly dissolved in water that has a little amount of sulfuric acid in an equimolar ratio to create Mohr's salt. The resultant solution is then put through a crystallisation procedure to produce light green Mohr's salt crystals. It should be noted that when salt is heated, it goes through an ionisation process that causes all the cations and anions it contains to be released. Manganese, lead, zinc, magnesium and nickel are typical contaminants that can be found in Mohr's salt. It is known that most of these contaminants can create isomorphous salts.
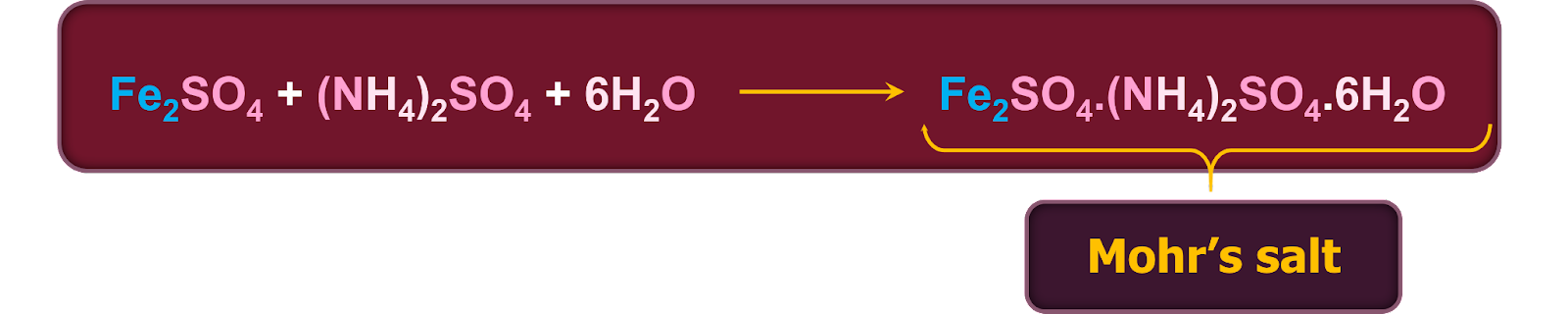
To stop ferrous sulphate from hydrolysing, typically, dilute sulphuric acid is added to stop the hydrolysis of ferrous sulphate. In general, excessive heating is avoided when dissolving the salt mixture in water. This is carried out in order to stop the light green Fe2+ ions from becoming Fe3+ ions (typically that are usually yellow in colour). If the yellow solution is produced, the procedure must be repeated. Mohr's salt crystals can be introduced into the concentrated solution to encourage crystal formation if the crystals do not separate after cooling. Typically, this is referred to as "seeding."
Mohr’s Salt – Properties
- Anhydrous Mohr's salt has the chemical formula , and its hexahydrate form has the chemical formula
- Anhydrous Mohr's salt has a molar mass of 284.05 gmol-1, and its hexahydrate form has the molar mass 392.13 gmol-1.
- Mohr's salt appears bluish-green in colour at standard temperature and pressure conditions, or STP for short. In these circumstances, it is a crystalline solid.
- At STP, Mohr's salt has a density of 1.86 g cm-3.
- The hexahydrate form of Mohr's salt dissolves in water at STP at a rate of about 269 gL-1.
Mohr’s Salt – Applications
Mohr's salt is among the most popular substances utilized as a source of Fe2+ ions (ferrous) in analytical chemistry. This solid has the benefit of having a reasonably long shelf life and being resistant to oxidation from exposure to the atmosphere, which makes it a good source of ferrous ions. When the pH of the surrounding is high (basic medium), it is known that the oxidation of this substance happens substantially more quickly. It is significant to note that the existence of ammonium cations in Mohr's salt solutions accounts for their typical mild acidity.
Sulphuric acid may be added to the solution of Mohr's salt to stop the ferrous ions from oxidising into ferric ions. Mohr's salt also plays a significant role in Fricke's dosemeter, which uses it to quantify high gamma radiation doses.
Practice Problems
1. When dissolved in water, Mohr's salt forms an aqua complex with the chemical formula __________ .
a. [Fe(H2O)6]3+
b. [Fe(H2O)6]2+
c. [Fe(H2O)6]1+
d. [Fe(H2O)2]2+
Answer: B
Solution: Mohr's salt dissolves in water to create an aqua complex with the chemical formula[Fe(H2O)6]2+. The molecular geometry of this aqua complex is octahedral.
So, option B is the correct answer.
2. Mohr's salt is a/an
a. Double Salt
b. Acidic Salt
c. Basic Salt
d. Normal Salt
Answer: A
Solution: Ammonium iron (II) sulphate, also known as Mohr’s salt is an inorganic salt that has the molecular formula FeSO4.(NH4)2SO4.6H2O. It contains two cations namely ammonium (NH4+) and ferrous (Fe2+). It is a double salt of ferrous sulphate and ammonium sulphate.
So, option A is the correct answer.
3. What is the oxidation state of iron in Mohr’s salt?
a. 0
b. +1
c. +2
d. +3
Answer: C
Solution: The chemical formula of Mohr’s salt is .
Consider the oxidation of Fe to be ‘x’.
The oxidation number of NH4 is +1
The oxidation number of SO4 is −2
The oxidation number of H is +1
The oxidation number of O is −2
The oxidation number of Fe in is calculated as,
Hence, the oxidation state of iron is +2 in
So, option C is the correct answer.
4. What is seeding?
Solution: Mohr's salt crystals can be introduced into the concentrated solution to encourage crystal formation if the crystals do not separate after cooling. This process is called seeding. Seeding is the addition of a pure crystal into its saturated solution in order to include and enhance crystallisation.
Frequently Asked Questions – FAQ
1. Mohr’s salt is acidic or basic?
Answer: Mohr’s salt is mildly acidic in nature. The existence of ammonium cations in Mohr's salt solutions accounts for their typical mild acidity.
2. What are the health hazards of Mohr’s salt?
Answer: Exposure to Mohr’s salt irritates the skin and eyes. If swallowed, Mohr’s salt causes nausea and vomiting.
3. How can we prepare Mohr’s salt solution?
Answer: Ammonium sulphate and hydrated ferrous sulphate can be dissolved in a solution of water and dilute sulphuric acid to create Mohr's salt in a laboratory setting. It is essential that ferrous sulphate and ammonium sulphate are taken in equimolar amounts.
4. Why is sulphuric acid added to Mohr’s salt solution?
Answer: To stop ferrous sulphate from hydrolysing, typically, dilute sulphuric acid is added to stop the hydrolysis of ferrous sulphate. In general, excessive heating is avoided when dissolving the salt mixture in water. This is carried out in order to stop the light green Fe2+ ions from becoming Fe3+ ions (typically that are usually yellow in colour).


