-
Call Now
1800-102-2727
Meso Compounds – Definition, Identification and Examples of Meso Compounds, Practice Problems and FAQ
Life that is both vibrant and gorgeous abounds throughout nature. For example, consider butterflies. We shall stop thinking about anything and start adoring these vibrant winged insects. In addition to being beautiful, nature may inspire us and help us understand some important concepts that would otherwise be difficult for us to picture. Meso compounds are one of these fascinating concepts. As a meso complex is divided into two identical parts by a centre of symmetry, it is comparable to a butterfly.

A meso compound can be separated into two equal halves by drawing a line vertically from top to bottom through the complex. When one is turned about 180 degrees, we can see that the two sections are identical since they will be a mirror image of one another.
We will learn more about meso compounds, their examples, and how to identify them in this article.
TABLE OF CONTENTS
- Meso Compounds
- Meso Compounds – Examples
- Meso Compounds – Identification
- Practice Problems
- Frequently Asked Questions – FAQ
Meso Compounds
A meso compound is an achiral compound with chiral centres. Although it has two or even more stereocenters, a meso compound has an interior plane of symmetry that renders it optically inactive and superimposable with its mirror image. A meso compound is a stereoisomer with two or even more chiral centres but does not show optical activity due to the presence of an internal plane of symmetry or centre of symmetry. Compounds known as mesomers have no net rotation in the plane of polarised light because there would be internal compensation of optical rotation. In other words, mesomers are organic compounds with zero net rotation and typically two identical chiral carbons.
Meso Compounds – Examples
Meso compounds feature chiral centres (sp3 hybridised tetrahedral atoms attached to four different groups), yet they are achiral generally due to the presence of elements of symmetry. As previously stated, a meso compound must include two or more chiral sp3 hybridised atoms with the exception of the nitrogen atom of a tertiary amine and at least one internal plane that creates two mirror images of the molecule. Tetrahedral centres are involved, hence the molecule can't be planar. Any compound which includes even one symmetry element is achiral, i.e. it won’t rotate plane polarised light. Meso compounds lack optical activity as a result.
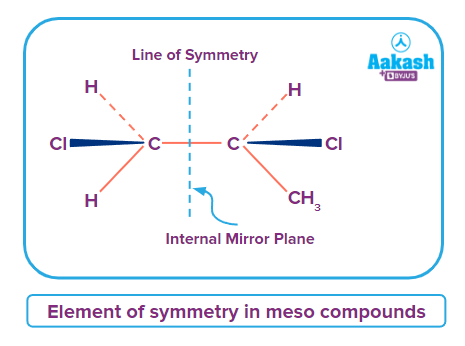
This molecule is achiral because it has a plane of symmetry. Nonetheless, it has two chiral carbons and is thus a meso complex. A non-optically active member of a collection of stereoisomers, of which at least two centres are optically active individually, is known as a meso compound or meso isomer. This indicates that the molecule lacks chirality while having two or even more stereogenic centres. They have numerous chiral centres because they are achiral compounds. This compound has an internal symmetry plane that divides it into two equal parts. The inner mirror allows these two sides to reflect one another. Therefore, the conclusion is that the chemical is optically inactive. Theoretically, cyclic molecules can also be meso compounds. Meso compounds do not exhibit optical activity because their optical activity cancels out in the presence of a plane of symmetry.
Achiral Diastereomers (Meso compounds)
In tartaric acid, the two chiral centres of the molecule 2,3-dihydroxybutanedioic acid are equal. Only three stereoisomeric tartaric acids are left once two out of the four probable stereoisomers of such a compound are determined to be equivalent by an axis of symmetry. Salts of tartaric acid were useful in deriving some of the subtleties of stereochemistry. A chiral diastereomer of 2,3-dihydroxybutanedioic acid would be a meso compound. Meso compounds are diastereomers with two of their stereoisomers being enantiomers and having chiral stereoisomers that are achiral (optically inactive). The physical characteristics of the several tartaric acid isomers are listed below.
(–)-tartaric acid: [α] D = –130
(+)-tartaric acid: [α] D = +130
meso-tartaric acid: [α] D = 00
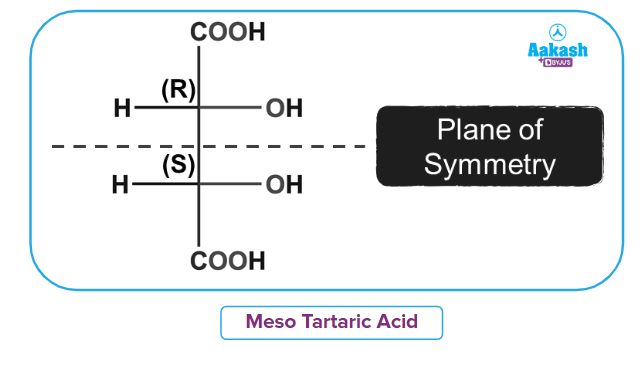
Fischer projection formulas can be used to visualise the configurational interactions that exist inside the geometries of such isomers. A Fischer projection formula can be rotated 180 degrees, typically in the plane. There is a mirrored line between mirror-image equations.
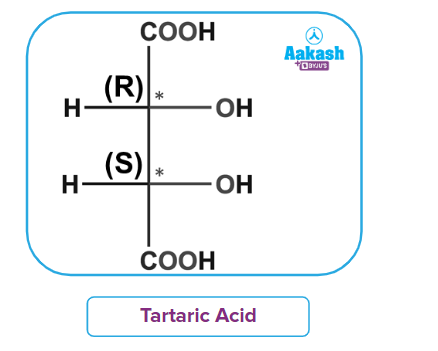
It contains 2 chiral centres but is still optically inactive due to the presence of a plane of symmetry.
After 180rotation in the plane of the screen
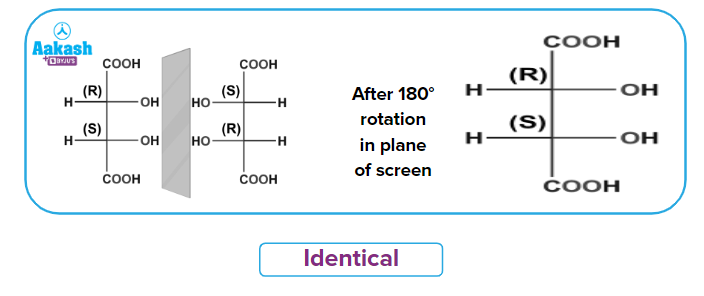
Therefore, mirror images are also superimposable. Internal compensation is what results in optical inactivity.
Meso Compounds – Identification
A meso compound must have two stereocenters or more, an inner plane, and stereochemistry for both R and S. To determine if a molecule is a meso compound or not, its stereochemistry (for instance, R or S) is crucial. Between the complexes, search for an inner mirror or plane. So that its optically inactive stereochemistry cancels out. When there are two stereocenters in a meso compound, R, for instance, cancels out S. A notable characteristic of single bonds or sp3-orbitals is the ability to determine the interior plane by rotating the substitution groups connected to the stereocenter. A molecule's stereochemistry is unaffected by rotation. There are still both meso molecules in place. Remember that the internal plane is now depicted in two dimensions. However, bear in mind that it is three-dimensional when looking for the internal mirror.
Recommended Videos
Optical isomers - Enantiomers | CHEMISTRY | Class 11/12 | NEET 2021/2022 | SM Sir
Enantiomers and Diastereomers | Isomerism | JEE 2023 Concept | Chemistry
Practice Problems
1. Which statement best describes meso compounds?
a. A chiral molecule having chiral centres
b. a chiral molecule that contains more than one chiral centres
c. a chiral molecule that contains more than one chiral centre
d. An achiral molecule that contains chiral centres
Answer: D
Solution: A meso compound is an achiral compound with chiral centres. Although it has two or even more stereocenters, a meso compound has an interior plane of symmetry that renders it optically inactive and superimposable with its mirror image. A meso compound is a stereoisomer with two or even more chiral centres but does not show optical activity due to the presence of an internal plane of symmetry or centre of symmetry.
So, option D is the correct answer.
2. A compound with asymmetric carbon atoms that can have its molecules superimposed on their mirror images is known as
a. a threo compound
b. a meso compound
c. an unsymmetric compound
d. an erythro compound
Answer: B
Solution: Meso compounds have asymmetric carbon atoms and their molecules are superimposable on their mirror images. Despite having asymmetric C atoms, meso compounds lack optical activity due to superimposable mirror images or other elements of symmetry. If two (or more) chirality centres in a molecule share the same set of four substituents, meso compounds may be formed. You might find it useful to examine molecular models when analysing stereoisomers.
So, option B is the correct answer.
3. The arrangement is ___________ if we move our eyes counterclockwise from the ligand with the highest priority to the ligand with the lowest priority.
a. R-Configuration
b. S-Configuration
c. E-Configuration
d. C-Configuration
Answer: B
Solution: Our eyes go counterclockwise from the ligand with the highest priority to the ligand with the lowest priority in the S-Configuration. Here is an example depicting the same.
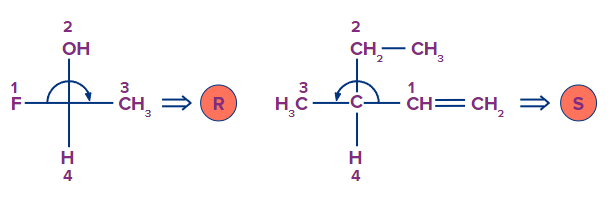
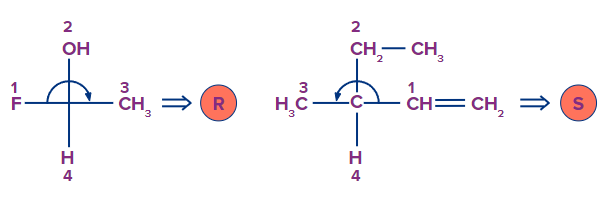
So, option B is the correct answer.
4. Which of the following is untrue about a meso compound?
a. The specific rotational angle is 0°
b. There are one or more symmetry planes
c. A single molecule and its mirror image are the same.
d. For each stereogenic centre, the stereochemical labels (R) and (S) must match exactly.
Answer: D
1. The specific rotational angle for meso compounds would be zero, as it would be optically inactive and hence would not rotate the plane polarised light. Therefore, the statement given in option A is true about meso compounds.
2. The molecules in meso form have a plane of symmetry as a result of which the optical rotations of the top and lower parts are equal and in the opposite direction, balancing internally and making the compound optically inactive. Due to the presence of two chiral carbons, the meso compound has a plane of symmetry (one with an R configuration and the other with an S configuration). Therefore, the statement given in option B is true about meso compounds.
3. A meso compound is superimposable to its mirror counterpart, indicating that the two structures are representations of the same molecule. Therefore, the statement given in option C is true about meso compounds.
4. For meso compounds, the stereochemical labels, (R) and (S), must be the opposite. If they have the same stereochemical labels, they are optically active and are not meso compounds. Therefore, the statement given in option D is untrue about meso compounds.
So, option D is the correct answer.
5. Which of the following groups, according to the Cahn-Ingold-Prelog sequence rules, has the highest priority?
a. CH2Cl
b. CHO
c. C2H5
d. CH2OH
Answer: A
Solution: The Cahn-Ingold-Prelog sequence rules state that the group with the higher atomic number has higher priority. Therefore, the order of priority is: CH2Cl > CHO > CH2OH > CH3.
So, option A is the correct answer.
Frequently Asked Questions – FAQ
1. Why are meso compounds achiral?
Answer: Meso compounds are achiral because they have a plane of symmetry and this will lead to a mirror image which is superimposable to the original molecule.
2. Are achiral compounds always meso?
Answer: A meso compound contains a plane of symmetry and so is achiral, regardless of whether the molecule has a chiral centre. A molecule is divided in half by a plane of symmetry, producing two halves that are mirror images of one another. Achiral molecules are not always meso compounds.
3. How can you tell whether a compound is optically active?
Answer: Optically active chemicals are those that have the ability to rotate optically. The optical activity of chiral substances is universal. The chiral chemical has an asymmetric core where four distinct atoms or groups are bonded to the carbon. It creates two mirror images that cannot be superimposed.
4. What are meso compounds in organic chemistry?
Answer: A stereoisomer set with at least two optically active members includes a non-optically active member called a meso compound or meso isomer. In spite of having two or even more stereogenic centres, the molecule is not chiral, according to this. A form of an element that cannot demonstrate optical activity as a result of dextrogyrate and levogyrate effects that are balanced contrary to each other in a structure.


