-
Call Now
1800-102-2727
Locant: Introduction of IUPAC Nomenclature, Assigning Locants, Examples, Practice Problems & Frequently Asked Questions
You might have booked your ticket for a cinema hall, railway or bus travel. When you enter the place, you are asked for the seat number. You occupy the seat already written there in some order. The seat number is your identity or position as long as you occupy the seat. Your name or qualification, and other identifications, all come later.
In your classroom or exam hall, roll numbers are written on the bench in some order, say starting from either the right or left end of the hall from your teacher’s podium and you occupy them as per your hall ticket number. The examiner can locate you from the seat number or locant.
In the same way, in an organic molecule, all the carbon atoms forming the longest chain length are numbered in an order given by the IUPAC Nomenclature system for the naming of an organic molecule. Atoms other than hydrogen attached to each of the chains are considered substituents. The number notifies the positions of the substituents in the organic molecules of the carbon atom to which they are attached. This carbon number of the attachment is called a locant, which specifies the position in the given carbon chain of the molecule.
In this article, we will discuss the functional group, locants and examples of locants in detail.
Table of Contents:
- Introduction of IUPAC Nomenclature
- Assigning Locants
- Examples of Assigning Locants
- Practice Problems
- Frequently Asked Questions(FAQs)
Introduction of IUPAC Nomenclature:
Simple organic compounds containing a few carbon atoms are still known by their common names.
Nevertheless, the IUPAC nomenclature provides the guidelines for assigning names for all organic molecules (about 10 million)
This consists of two parts-
- Writing the name of the molecule from its constituents of hydrocarbon and the substituents on it.
- Assigning locants(numbers) to indicate the positions of the substituents on the hydrocarbon chain.
Guidelines for Naming the Organic Molecule.
The format of the organic molecular name is:
Secondary prefix + Primary prefix + Word root + Primary suffix + Secondary suffix
Secondary prefix - are from the substituents (except the highest priority group) on the parent chain written in alphabetical order of their first few letters. Substituents like a halo, nitro, and Alkyl /aryl are always written as secondary prefixes.
Primary prefix - is the nature of the parent chain (cyclo, bicyclo, spiro)
Root Word - is from the hydrocarbon that contains-
i) longest chain of carbon atoms in the molecule
ii) the highest priority group if any in the hydrocarbon chain
iii) the most number of unsaturated bonds in the chain
iv) most number of substituents in the molecules.
The order of selection of parent chain is given as follows:
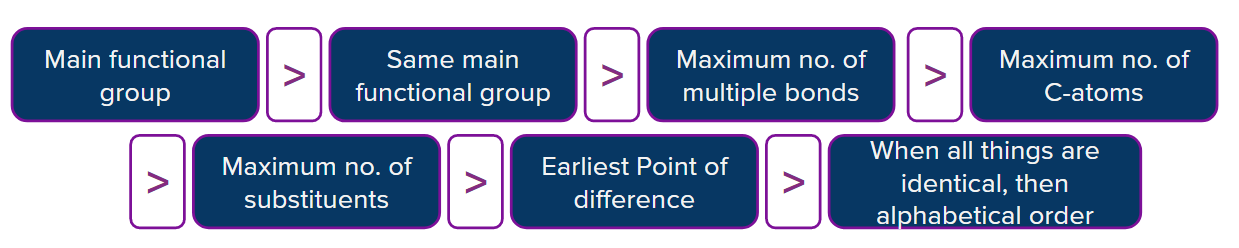
Primary Suffix - Ending of the hydrocarbon indicating the level of saturation or unsaturation of the parent
Chain- ‘ane,’’ene’ and ‘yne’ in the same order.
Secondary Suffix - is taken from the most priority functional group present in the molecule.
The following table lists functional groups in decreasing order of priority. They must be taken into account when numbering.
|
Class |
Name |
Secondary Prefix |
Secondary Suffix |
|
R-COOH |
Carboxylic acid |
carboxy |
-oic acid |
|
R-SO3H |
Sulphonic acid |
sulpho |
-sulphonic acid |
|
(R-CO)2O |
Acid anhydride |
- |
-anhydride |
|
R-COOR |
Ester |
carboalkoxy |
-oate |
|
R-COX |
Acid halides |
halo carbonyl |
-oyl halide |
|
R-CONH2 |
Amides |
carbamoyl/carboxamide |
-amide |
|
R-CN |
Cyanide |
cyano |
-nitrile |
|
R-CHO |
Aldehyde |
formyl/oxo |
-al |
|
R-COR |
Ketone |
keto/oxo |
-one |
|
R-OH |
Alcohol |
hydroxy |
-of |
|
R-SH |
Thiol |
mercapto |
-thiol |
|
R-NH2 |
Amines |
amino |
-amines |
|
R-OR |
Alkoxy |
- |
- |
|
-O- |
Epoxy |
- |
- |
IUPAC Nomenclature of polyfunctional group:
When an organic molecule has two or more different functional groups, choose the primary functional group based on the priority list. The other functional groups will be treated as substituents and a prefix will be given.
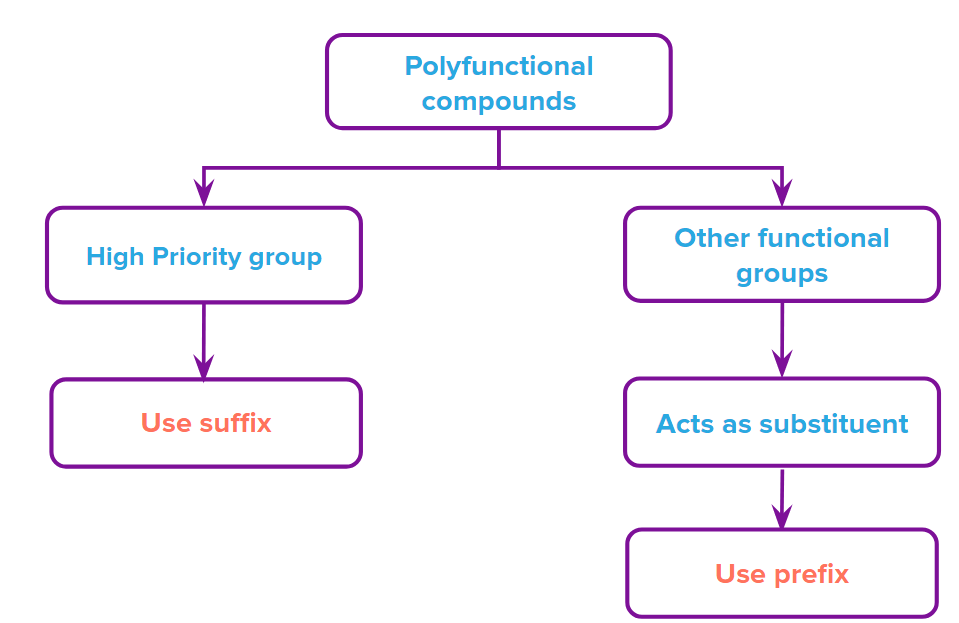
Assigning Locants:
The following guidelines are to be followed in assigning locants to the substituents.
1. Locants are integers starting from 1.
2. The first locant (1) starts from the
i) the end carbon of the longest hydrocarbon chain.
ii) carbon atom of the carbon containing primary functional group to be included in the longest chain.
3. Further, locant assignment goes with the aim of giving lowest locants to the substituents in the order of-
i) double bond followed by triple bonds
ii) at the first point of difference
ii) Overall locant should be minimum
Examples of Assigning Locants:
A) Saturated Hydrocarbons:
Example 1:
A pair of locants can be assigned by a number from either side of the chain.
In the first example given below, numbering from left gives the substituents locants of 2,4,6-trimethyl decane. On the other hand, numbering from right gives the locants as 5,7,9-trimethyl decane.
Since 2, 4, 6 is the lowest locant from both first point of difference and over all lowest locants than 5,7,9, the correct name will be 2,4,6-trimethyl decane.
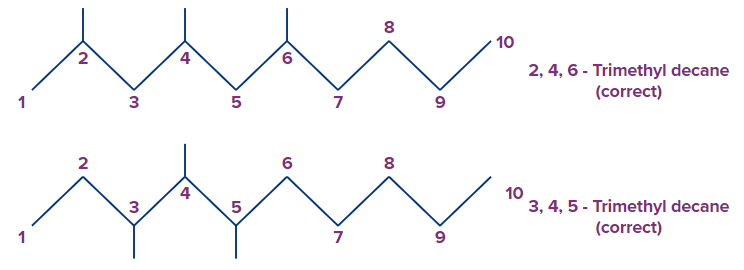
In the second example also we can have two numberings of locants as
3,4,5-trimethyl decane and 6,7,8-trimethyl decane. As per the lowest locant rule, 3,4,5-trimethyldecane is the correct name.
Example 2:
Here again the first point of difference in the following molecule should be 2 rather than 3. So the locants will be 2-methyl and 3-ethyl. Remember to write the prefixes in alphabetical order irrespective of the locant positions. As E comes before M in alphabetical order, ethyl is written first followed by methyl substituents.
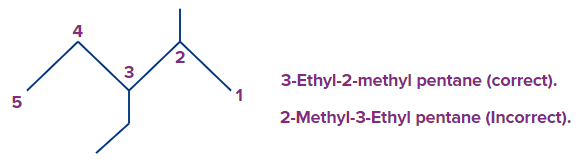
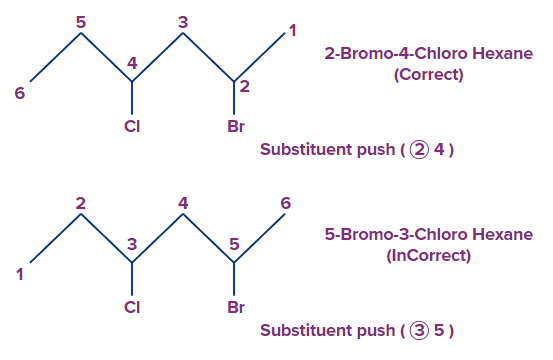
Example 3. Locants 2,4 is preferred to 3,5, for the substituents. So the correct name will be 2-Bromo-4-chlorohexane following the alphabetical order of substituents.
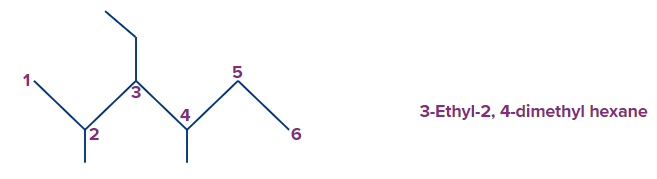
Example 4: Multiple numbers of the same substituents shall have corresponding prefixes such as, di, tri, tetra, etc.
In the given example methyl groups occur at 2 and 4 positions. Hence they are clubbed as di methyl with locant positions in front as follows.
Example 5. The below example has alkyl substituents at 3 and positions either way you number it. In such cases of the lowest set of locants, the rule is not applicable, alphabetical order is used for numbering. Hence ethyl group with ‘e’ first letter is given the lowest locant and the methyl group locant 5.
So, the correct name is 3-ethyl-5-methyl heptane is the right name.
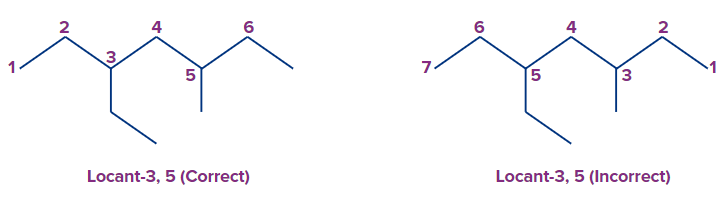
Example 6: If di, tri, tetra, and so on are part of the complex name, they are considered in alphabetical order.
In the below compound, the substituents at 3 and 5 are pure ethyl groups. The substituent at 4 is a complex substituted ethyl group - 1,1-dimethyl ethyl. This complex alkyl substituent is written first in the naming.

B) Unbranched Unsaturated Hydrocarbons:
Rule-1: Numbering is done from the side of the unsaturated carbon so as to give the lowest locant to the unsaturated carbon in the molecule.
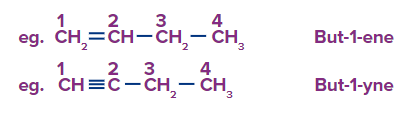
Rule 2: Numbering can be done either way if the terminal carbon atoms contain the same unsaturated double bond or triple bond.
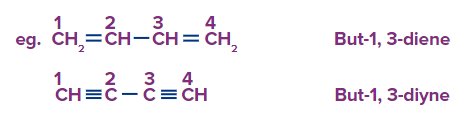
Rule 3: Numbering always starts nearer to the double-bonded terminal carbon atom if an unsaturated bond, such as a double bond or triple bond, is present at the terminal carbon atom. (When both bonds are in the same position, a double bond is preferred over a triple bond.)

Rule 4: If a double bond exists at the terminal carbon and a triple bond exists at any carbon other than the terminal carbon. Then numbering will start from a double bond.
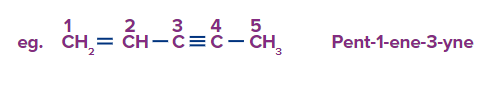
Rule 5: If a triple bond exists at the terminal carbon and a double bond exists at any carbon other than the terminal carbon. Then numbering will start from a triple bond so as to give minimum locants.
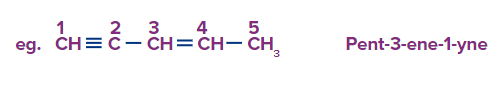
C) Branched Unsaturated Hydrocarbon:
Rule-1: Choose the parent chain of a branched alkene that has the most unsaturated double bond or triple bond, and then adhere by the lowest locant rule for the unsaturated carbons.
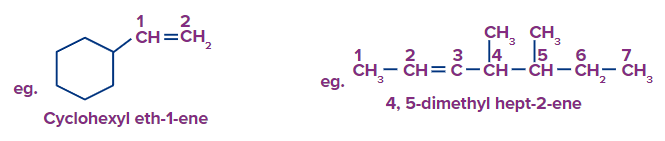
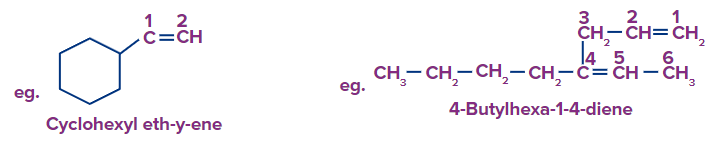
Note: Among the linear alkenes, the first compound parent chain contains 7 carbon atoms, without any ambiguity. But in the second structure, The longest carbon chain containing both double bonds is taken
Rule-2: In the case of an unsaturated cyclo compound with a side chain, numbering is done starting from the double bond carbon of the cyclic ring and adhering to the lowest locant rule..
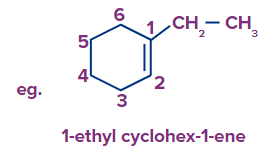
D) Multiple Substituents:
Example 1:
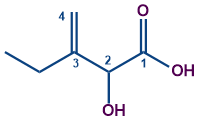
The molecule contains a carboxylic acid and a double bond. Including both carbons of carboxylic acid and the double bond, the longest hydrocarbon chain contains four carbons. The word root is ‘But’.
The functional group with the highest priority is a carboxylic acid. So the carboxylic acid group is the secondary suffix with the suffix of ‘oic acid’.
The double bond gives a primary suffix of ‘ene’.
The alcohol group will become the secondary. prefix with ‘hydroxy’ as a secondary prefix.
The ethyl group is the primary suffix.
Adding all of these, the name becomes Ethyl hydroxybutanoic acid.
Starting the locant from the suffix carbon and ending at the double bonded carbon, we have hydroxy at position 2 and ethyl group at position 3. The double bond also occurs at position 3.
It should be remembered that a hyphen will separate the locants from the name.
So the compound IUPAC's name is 3-Ethyl-2-hydroxybut-3-enoic acid.
Example 2:
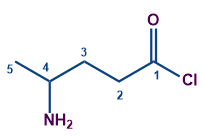
Longest chain contains 5 carbon atoms (including the carbon of acyl group) Word root - Pent
No unsaturation. So first suffix = Nil
The acid chloride group has the highest priority as per the priority table. So the secondary suffix is-oyl chloride
The compound is linear and so the first prefix = Nil
The amine is the substituent having a secondary prefix of amino.
Substituent amino is in the fourth carbon.
The compound IUPAC's name is 4-Aminopentanoyl chloride.
Example 3:
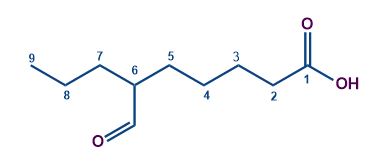
Longest chain contains 9 carbon atoms (including the carbon of the carboxylic acid group) Word root - Nonan
No unsaturation. So the first suffix = Nil
The acid group has the highest priority as per the priority table. So the secondary suffix is-oic acid
The compound is linear and so the first prefix = Nil
The aldehyde is the substituent having a secondary prefix of formyl.
Substituent formyl is in the sixth carbon.
So the compound IUPAC's name is 6-Formyl nonanoic acid.
Example 4:
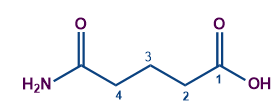
Longest chain contains 4 carbon atoms (including the carbon of the acid group) Word root - Butan
No unsaturation. So the first suffix = Nil
The acid group has the highest priority as per the priority table. So the secondary suffix is-oic acid
The compound is linear and so the first prefix = Nil
The amide is the substituent having a secondary prefix of carboxamide.
Substituent amino is in the fourth carbon.
So the compound IUPAC's name is 4-Carboxamide Butanoic acid.
Example 5:
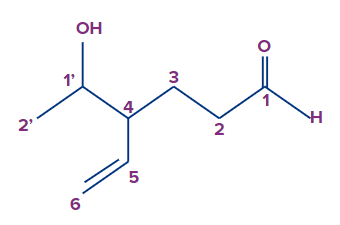
Longest chain contains 6 carbon atoms (including the carbon of aldehyde group) Word root - Hex
Unsaturation of a double bond. So first suffix = ene
The aldehyde group has the highest priority as per the priority table. So the secondary suffix is-al
The compound is linear and so the first prefix = Nil
The hydroxy-substituted ethyl with the substituent having a secondary prefix of 1-hydroxyethyl.
The substituent is in the fourth carbon.
So the compound IUPAC's name is 4-(1-hydroxyethyl)hex-5-enal.
E) IUPAC Nomenclature of Compounds with more of the same Principal Functional Group:
Example 1:
Carbon atoms of repeating terminal functional groups in a chain are excluded from the longest carbon length. The functional group is given a unique and common suffix.
Example 1: The terminal acid group is attached at three more places of the carbon chain. Leaving all the carboxylic carbon, the longest chain length is 7 and not 8 carbons as shown in the figure.
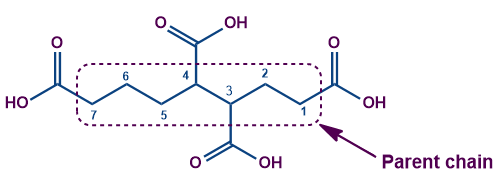
Here the parent chain consists of seven carbon atoms. The compound IUPAC's name is Heptane-1,3,4,7-tetracarboxylic acid.
Example 2:
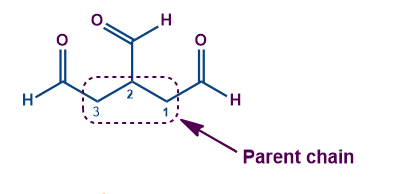
Here the parent chain consists of three carbon atoms and has three aldehyde functional groups, attached to the parent chain. Excluding them from the chain, the compound IUPAC's name is Propane-1,2,3-tricarbaldehyde.
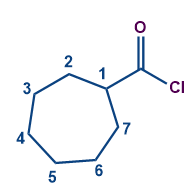
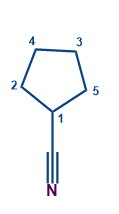
F) IUPAC Nomenclature of Cyclic Compounds having Functional Groups:
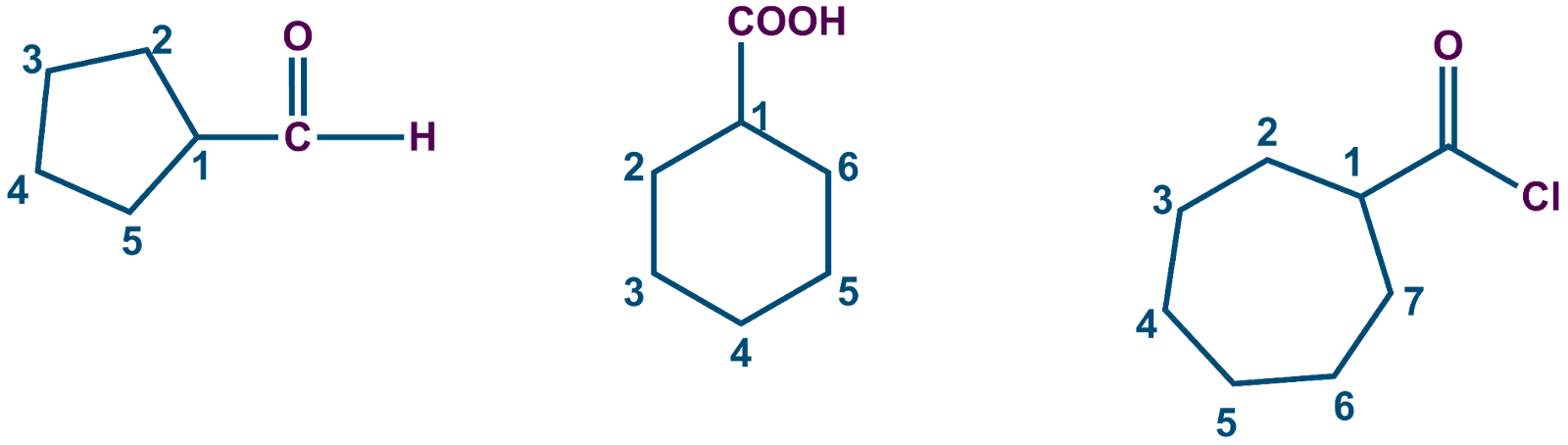
When a ring is directly joined to a functional group that contains carbon, the ring is referred to as the parent chain. These functional groups, which are listed as follows, are assigned a specific suffix:
|
Functional Group |
Special Suffix |
Example |
IUPAC Nomenclature |
|
-COOH |
Carboxylic acid |
|
Cyclohexane carboxylic acid |
|
-CHO |
Carbaldehyde |
|
Cyclopentane carbaldehyde |
|
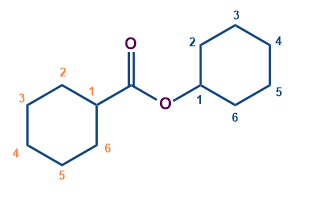
-COOR |
carboxylate |
|
Cyclohexyl cyclohexane carboxylate |
|
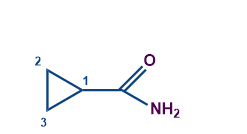
-CONH2 |
Carboxamide |
|
Cyclopropanecarboxamide |
|
-COX |
Carbonyl halide |
|
Cycloheptane carbonyl chloride |
|
-CN |
Carbonitrile |
|
Cyclopentanecarbonitrile |
Practice Problems:
Q1. What is the following compound's IUPAC name?
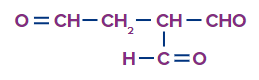
(A) Ethane-1,1,2-tricarbaldehyde (B) 1,1-diformyl propanal
(C) 3-formyl butanedial (D) 2-formyl butanedial
Answer: (A)
Solution: The numbering of the compound is given as
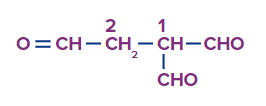
Two carbon atoms constitute the parent chain in this case, however those three aldehyde functional groups are excluded from the parent chain, hence the chemical is given a special name. So, the IUPAC name for the compound is Ethane-1,1,2-tricarbaldehyde.
Q2. What is the correct numbering order in the given compound?
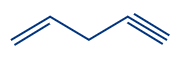
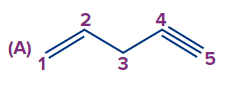
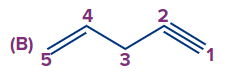
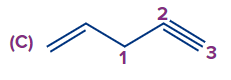
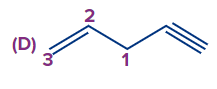
Answer: (A)
Solution: Numbering always starts at the double-bonded terminal carbon atom if an unsaturated bond, such as a double bond or triple bond, is present at the terminal carbon atom. (When both bonds are in the same position, a double bond is preferred over a triple bond). So the compound IUPAC's name is pent-1-ene-4-yne.
Q3. What is the correct numbering order in the given compound?
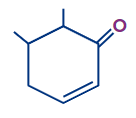
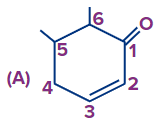
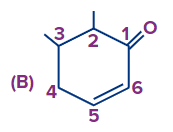
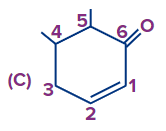
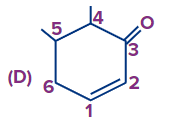
Answer: (A)
Solution: If a functional group and double bond are present then priority is given to the functional group and the functional group has to get the first number and after that double bond will be given the least possible number as per the above-discussed rules the correct number order of the structure is (A).
(Priority order: Functional Group > double/triple bond > substituent).
Q4. What is the following compound's correct IUPAC name?
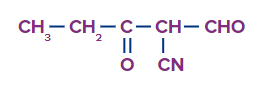
(A) 2-formyl-3-oxo pentanenitrile (B) 2-cyano-3-oxo pentanal
(C) 2-cyano -1,3- pentanedione (D) 1.3 dioxo-2-cyano pentane
Answer: (A)
Solution: Here the priority functional group is cyanide so the suffix used is nitrile and -CHO and CO acts as substituents and prefixes for these are formyl and oxo respectively.
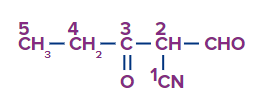
As per the numbering, the compound IUPAC's name is 2-formyl-3-oxo pentanenitrile.
Frequently Asked Questions(FAQs):
Q1. Name the functional groups that are always used as prefixes.
Answer: The functional groups that are always used as prefixes are halides (fluoro, chloro. Bromo and iodo), Nitro group (-NO2) and azide group (-N3).
Q2. Why are double bonds or triple bonds considered functional groups?
Answer: Atoms or groups of atoms inside a molecule that have locations of relatively high reactivity, such as double and triple bonds, are referred to as functional groups. Therefore, double or triple bonds represent functional groups as well.
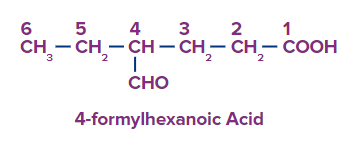
Q3. What is the difference between formyl and oxo prefixes in aldehydes?
Answer: If an aldehyde or ketone group is located on the longest continuous carbon chain that also contains a higher priority group, the substituent term for that group is oxo.
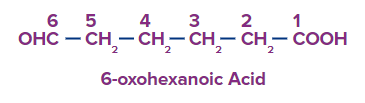
Aldehyde carbons become formyl substituents if they are not a component of the longest continuous chain.
Q4. Which functional group has more priority Ester or Acid halide?
Answer: One functional group is used as the major functional group and the other functional group is used as a substituent when a molecule has more than one functional group. Ester is given greater priority than acid halide when comparing the two.