-
Call Now
1800-102-2727
Law of Equivalence-Concept of Equivalence- Definitions, Example, Practice Problems & FAQ
How many of you have heard of “Ginger ale”?
It's a common drink used to aid digestion, and reduce nausea among other benefits. But do you know how “Ginger ale” is actually prepared?
The answer might surprise you.
It's actually a mixture of diet coke and sprite. But would just mixing the two drinks in any proportion suffice? Absolutely not. Ginger ale is made by mixing 70% sprite and 30% coke. So in a way you have to carefully administer the ratio in order to make the said drink. But what if one had no clue about the proportion? Wouldn't it be very difficult to make it? Yes. The task of knowing the ratio of ingredients is highly crucial in preparation. The same logic follows while we are utilising chemical reactions too.
Similarly if one asks how one can calculate the amount of product formed or the amount of a reactant consumed during a chemical reaction without balancing the reaction?
If a chemical reaction is balanced, it becomes effortless to do mole-mole analysis and you can easily find the mole of product formed or the mole of reactant consumed. The same information also can be drawn from any chemical reaction without balancing a chemical reaction or without writing the whole product or reactant of the reaction with the help of the law of equivalence.

Table of contents
- Law of equivalence
- Practice problems
- Frequently asked questions-FAQs
Law of equivalence
If you observe a balanced chemical reaction you can withdraw a piece of information in which molar ratio reactants are combined and products are formed.
Take for example the reaction of water production
![]()
we can say 1 mole of hydrogen gas combined with 0.5 moles of oxygen gas to form one mole of the water molecule.
The above-mentioned reaction could also be written as
2H2(g)+O2(g)2H2O(l)
We can say two moles of hydrogen gas combined with one mole of oxygen gas to form two moles of the water molecule.
The same molar ratio can be obtained for an unbalanced reaction without knowing the stoichiometric coefficient of compounds involved in a chemical reaction with the help of the n-factor or valency factor.
It states that one equivalent of an element always combines with one equivalent of other elements. Or In a chemical reaction, the equivalents or milliequivalents of the reactants react in equal amounts to give the same number of equivalents or milliequivalents of the products separately.
For a chemical reaction, n1A+n2Bn3C+n4D
Where n1, n2, n3, and n4 are the stoichiometric coefficients in a balanced chemical reaction
According to the law of equivalence,
Equivalents of A consumed = Equivalents of B consumed = Equivalents of C formed = Equivalents of D formed
We can see, the number of equivalents of a substance is n-factor multiplied by the number of moles or Normality of solution multiplied by volume in litre.
For a neutralization (acid-base) reaction:
At the equivalence point,
⇒Equivalent of Acid = Equivalent base
For a redox reaction:
At the equivalence point,
⇒Equivalent of oxidizing agent = Equivalent of reducing agent
Therefore, we can say that in a reaction, the number of moles of reactants and products may or may not be equal, but the number of gram equivalents will always be equal.
example: 2Fe(s)+3Cl2(g)2FeCl3(s)
Practice problems:
Q 1. Calculate the amount of oxygen required to produce enough carbon monoxide on reaction with carbon which can reduce 800 g of Fe2O3.
a. 420 g
b. 840 g
c. 220 g
d. 110 g
Answer: (A)
Method 1: by mole-mole analysis
First write a balanced equation: Fe2O3+3CO2Fe+3CO2
From the balanced reaction we can see, 1 mole of Fe2O3 reduced by 3 moles of CO.
Molar mass of Fe2O3=160 g mol-1
Molar mass of CO=28 g mol-1
160 g Fe2O3 can be reduced by 84 g of CO
800 g Fe2O3 can be reduced by (84160800)= 420 g of CO
Method 2: by the law of equivalence
Fe2O3+COFe+CO2 (unbalanced)
By the law of equivalence; the equivalent of Fe2O3 = equivalent of CO
moles of Fe2O3 n-factor of Fe2O3 = moles of CO n-factor of CO
Molar mass of Fe2O3=160 g mol-1
Molar mass of CO=28 g mol-1
Let, the weight of required CO=x g
![]()
x= 420 g
Q 2. Find the number of moles of oxalate ions that are completely oxidized by one mole of MnO4- ion in an acidic medium.
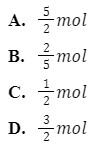
Answer: (A)
We should know the products of some famous oxidizing and reducing agents in acidic and basic mediums.
E.g:
In acidic medium: MnO4-Mn2+
In highly basic medium: MnO4-MnO42-
In slightly basic or neutral medium: MnO4-MnO4
In acidic medium: Cr2O72-Cr3+
![]()
For this question, in acidic medium

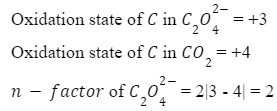
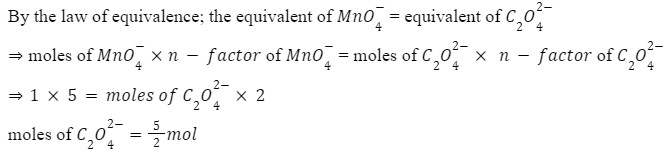
Q 3. How many moles of potassium permanganate are needed to completely oxidize a mixture of two moles of ferrous sulfate and three moles of ferrous oxalate in an acidic medium
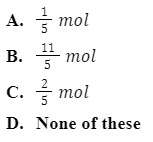
Answer: (B)
Write the chemical reaction:


Q 4. Select incorrect statement

Answer: (A)
for monobasic acid n-factor = 1
So, we can write normality = molarity
Frequently asked questions-FAQs
Q 1. What is equivalent weight?
Answer: The number of parts by mass of an element that reacts or displaces from a compound 1.008 parts by mass of hydrogen or 8 parts by mass of oxygen or 35.5 parts by mass of chlorine, is known as the equivalent weight of that element.

Q 2. Find the molar ratio if two reactants combine together and their ratio of n-factor is x:y
Answer: When two reactants combine in a way that their n-factors are in the ratio of x:y, they always combine with each other in the molar ratio of y:x in a balanced chemical reaction.
Q 3. What is n-factor ?
Answer: n-factor of an acid is the number of H+ ion furnished per molecule of acid and for a base, number of OH- ion furnished per molecule of the base.
Q 4. Equivalent weight depends on which factors?
Answer: Equivalent weight depends on molar mass and n-factor of the compound. n-factor is decided by the type of reaction involved. n-factor depends upon the number of ionizable protons (in case of an acid-base reaction) or on the number of electrons lost/gained (in case of redox reactions).
Related Topics:
|
Volume strength of H2O2 |
Mole |
|
Strength of oleum |
ppm |
|
Strength of solution |
Density |


