-
Call Now
1800-102-2727
Lattice Point: Definition of Lattice Point, Bravais Lattice & Seven Crystal System, Practice Problems & FAQs
Imagine, someone asked you to prepare a map of your locality on paper. The map should include all the residential places, roads, fuel stations, hospitals and everything that you see on a google map.
What will be your approach?
Well, it's a tough job, but someone has to do it. Obviously, drawing all the residential places exactly the way they look won’t be possible. Instead, you can draw dots to represent residential places. Did I just make your job easier? You can thank me later.
Similarly, in chemistry the structure of a solid compound is generally understood through a crystal lattice and the position of atoms, ions or molecules is represented by a dot called lattice points.
In this concept page, we will explore lattice points and the seven crystal systems!
TABLE OF CONTENT
- Definition of Lattice Point
- Types of lattice
- Seven Crystal system and Bravis lattices
- Practice problems
- Frequently Asked Questions-FAQs:
Definition of Lattice Point:
A crystal lattice is a solid substance whose constituent particles (atoms, molecules, or ions) are arranged in an organized pattern that spans all three spatial dimensions.

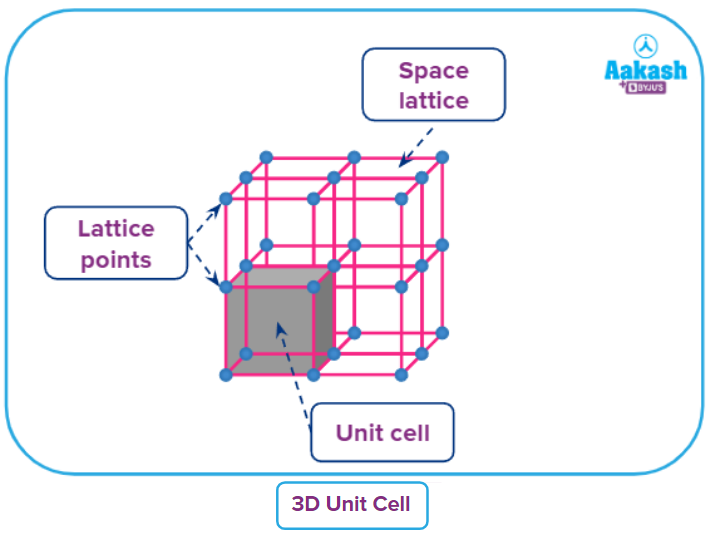
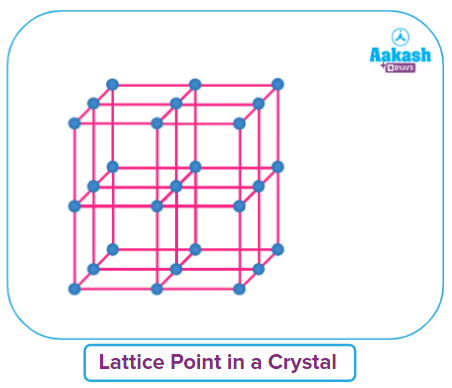
In a crystal, the lattice point is generally represented by a point/dot ( . ), symbolizing the center of an atom, ion, or molecule. In a crystal lattice, the connecting points are all connected by straight lines. The structure can be seen in three dimensions by joining these straight lines. Crystal lattice, also known as Bravais lattice, is the name of this three-dimensional structure.
Types of lattice:
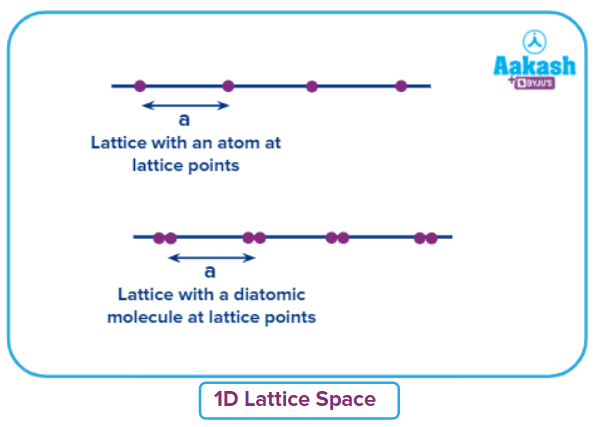
1-D (One-dimensional) space lattice:
- If we arrange atoms/ions touching each other in a line (one dimension), Lattice points are uniformly separated in a one-dimensional space lattice.
- Only one parameter (edge length - a) is required to define a 1-D space lattice and that is the distance between any two consecutive lattice points. Requirements to fix the arrangement is called a lattice parameter. In one dimensional lattice the lattice parameter is only one, namely length.
- Repeating units can be observed at every lattice parameter. The unit is the basic or fundamental unit of the entire lattice like a polymer made of monomer in one direction.
- In a 1-D space lattice, lattice points can be either occupied by a single
- atom/ion (case A) or by a polyatomic molecule (case B).
2-D (Two-dimensional) space lattice:
● Regular arrangement of lattice points in a plane(on a surface) gives a two-dimensional space lattice.
● The lattice is defined by three parameters: two edge lengths a and b, and the angle between them .
3-D (Three-dimensional) space lattice:
● The regular arrangement of lattice points in space will give a three-dimensional space lattice.
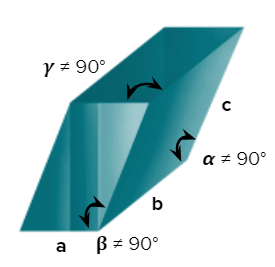
● The lattice points in space can be fixed In terms of the six lattice parameters (3 lengths and 3 angles). The crystalline solid repeats the small unit with the lattice parameters in three dimensions. The small repeating unit repeating to make the entire crystal is called unit cell.
The lattice parameters in 3D space lattice can be arranged in seven unique unit cell shapes known as crystal systems. The crystal systems have varying elements of symmetry in a three-dimensional space.
Seven Crystal system and Bravis lattices:
Almost all the crystals found in nature conform to one of these seven crystal systems. The basic repetitive units present in these systems are of 14 types and called Bravais lattice systems. As a whole the unit cell of all the solid shall fall under any of these 14 Bravis lattice types with differing lattice parameters.
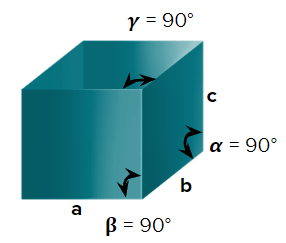
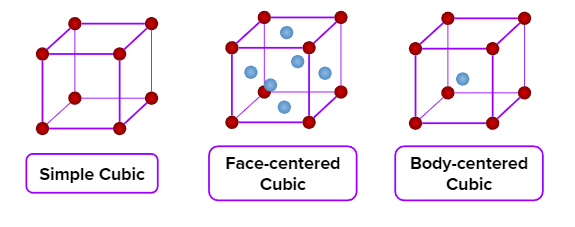
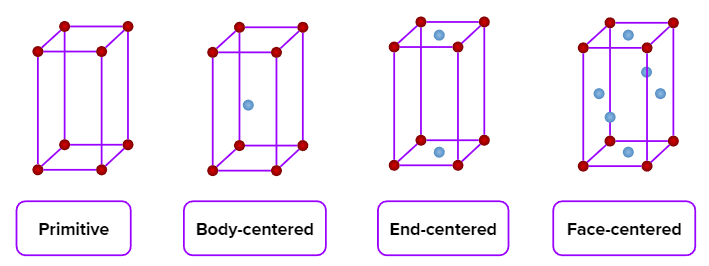
- Cubic
- Axial Length : a=b=c & Axial angle:
- NaCl, ZnS, Cu, KCl, Alums, Diamond, Zinc blende
- Simple cubic (primitive), body-centred cubic, and face-centred cubic unit cells are possible in a cubic crystal system.
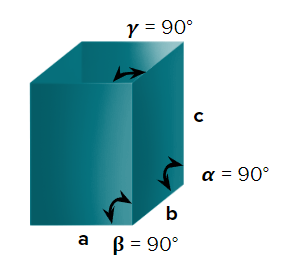
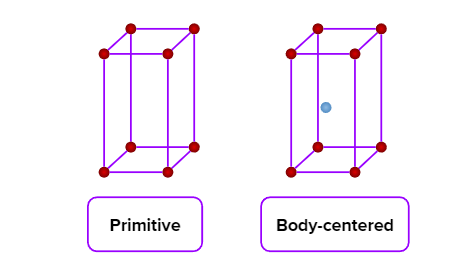
- Tetragonal
- Axial Length: & Axial angle:
- White tin(Sn), SnO2,TiO2,CaSO4
- Simple (primitive) and body-centred unit cells are possible in a tetragonal crystal system.
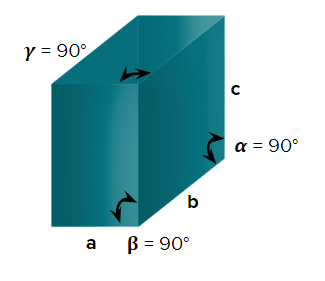
- Orthorhombic or Rhombic
- Axial Length: & Axial angle:
- KNO3,K2SO4,BaSO4, PbCO3, CaCO3Rhombic sulfur
- Simple (primitive), end-centred, body-centred, and face-centred unit cells are possible in an orthorhombic crystal system.
- Monoclinic
- Axial length: & Axial Angle:
- Na2SO4. 10H2O,PbCrO4, Monoclinic Sulfur
- Simple (primitive) and end-centred unit cells are possible in a monoclinic crystal system.

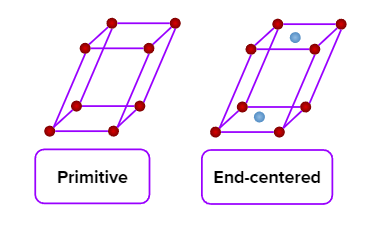
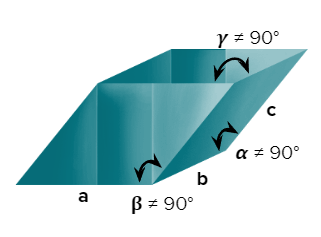
- Triclinic
- Axial Length: abc & Axial angle: 900
- CuSO4, 5H2O, K2Cr2O7, H3BO3
- Only a simple (primitive) unit cell is possible in a triclinic crystal system.
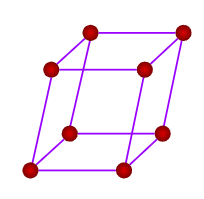
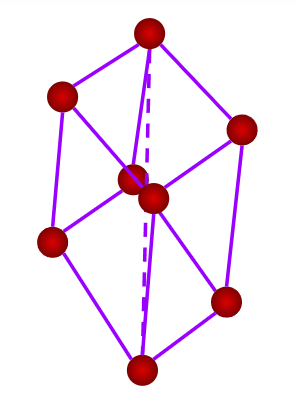
- Rhombohedral or Trigonal
- Axial Length: a=b=c & Axial angles:
- NaNO3, ICl, As, Sb, Bi, Calcite(CaCO3)
- Only a simple (primitive) unit cell is possible in a rhombohedral crystal system.
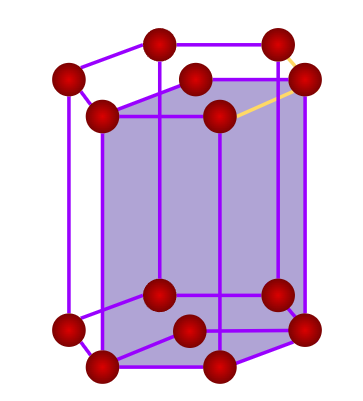
- Hexagonal
- Axial Length: & Axial Angle:
- Cinnabar(HgS), Ice Graphite, Mg, Zn, Cd, ZnO, CdS, AgI, PbI2
- Only a simple (primitive) unit cell is possible in a hexagonal crystal system.

Recommended Video: Crystal Lattice and Unit Cell Class 12 Chemistry - Solid State Concepts (L 2) | NEET 2023 Chemistry
Practice problems:
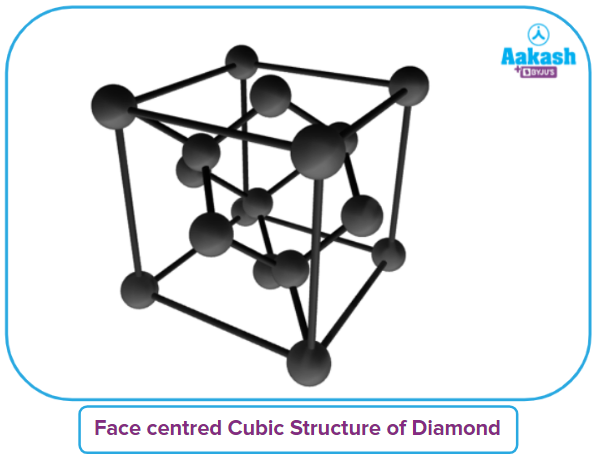
Q1. Crystal of diamond crystallizes in which type of lattice structure?
a. FCC
b. BCC
c. ECC
d. None of the above
Answer: (A)
Solution: Diamond is an allotrope of carbon where one carbon atom is connected to 4 other carbon atoms through covalent bonding. Hence, diamond is a covalent crystalline solid. The hybridization of carbon atoms in diamond is sp3. Each carbon atom is tetrahedrally arranged to form a giant network having FCC arrangement.
Q2. The lattice in a pure crystal can’t be occupied by:
a. Molecule
b. Ion
c. Electron
d. Atom
Answer: (C)
Solution: Electron is a subatomic particle that is present inside the atom. In the case of a pure crystal, all the lattice points are generally occupied by an atom, molecule, or ions.
Q3. What is the coordination number of atoms in a BCC?
a. 1
b. 8
c. 2
d. 4
Answer: (B)
Solution: The coordination number of an atom in a crystal is the number of constituent particles which are the immediate neighbors of that atom in a crystal. The coordination number of an atom in BCC is 8.
Q4. Which of the parallelograms in the figure given below can be taken as unit cells?
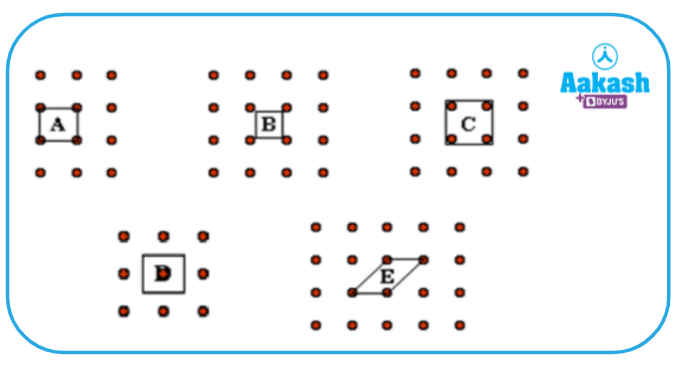
a. Only A
b. A, B and C
c. A and D
d. A, D and E
Answer: (D)
A unit cell is the basic repeating structure of any solid, when they are repeated it forms a crystal lattice.
In the case of ‘A’ when repeated can form the crystal lattice.
In the case of ‘B’ when repeated it can not form the crystal lattice as atoms are outside the unit cell.
In the case of ‘C’ when repeated it can not form the crystal lattice as atoms are entirely inside the unit cell. If we repeat this unit cell, the structure which we will get is different from the original one.
In the case of ‘D’ when repeated can form the crystal lattice as it contains only one atom inside so it will form the proper crystal structure.
In the case of ‘E’ when repeated can form the crystal lattice as ‘E’ unit cell is just a lateral shape of ‘A’.
Frequently Asked Questions-FAQs:
1. State the difference between crystal lattice and unit cell.
Answer:
|
Crystal Lattice |
Unit Cell |
|
Crystal Lattice can be defined as the three-dimensional geometrical arrangement of constituent particles in a solid. |
It is the smallest repeating portion of a crystal, which is arranged in a repeated pattern to form a crystal structure or crystal lattice. |
2. Do all solids form crystal lattices?
Answer: No, crystalline solids have long-range order whereas in the case of amorphous solids the arrangement of particles has a short-range order. Hence, the crystal lattice is only found in crystalline solids.
3. Are the space lattice and unit cell the same?
Answer: No, space lattice is the three-dimensional arrangement of crystals that are constituted by unit cells. In other words, we can say that unit cells arrange themselves in a repeating pattern to create a space lattice.
4. What exactly is the lattice point in a crystal structure?
Answer: In simple words lattice points are coordinates or positions where the particle resides. These lattice points are arranged in an ordered fashion to give a lattice structure.