-
Call Now
1800-102-2727
Lattice Energy – Definition, Factors Affecting Lattice Enthalpy, Types and Calculation of Lattice Energy
Look at how this toddler is playing with his favourite geoboard! It's a wooden board consisting of wooden nail-like pillars with threads or rubber bands wrapped around the nails in a way that connects these points present on the board, to form various geometrical shapes– such as triangles, rectangles, squares etc.
Isn’t this fascinating how the points on the geoboard are connected by the colourful rubber bands, thereby constructing a meaningful shape!
Truly so and thus ‘geoboards' are a popular manipulative method for introducing basic mathematical concepts of plane geometry and various polygons.

The visuals on this geoboard are to some extent analogous to an ionic lattice! The wooden nails can be considered as a representation of ions of a compound and the rubber bands connecting them are like the electrostatic forces of attraction connecting the oppositely charged ions, thereby resulting in the formation of an ionic crystal or ionic lattice. This kind of arrangement that you can perceive in the geoboard, is actually a basic representation of an ionic lattice. The force (connective rubber bands) which binds the wooden nails (ions in case of a compound) together forming this beautiful lattice structure, is what we consider as ‘Lattice Enthalpy’
Let’s now dig deeper into this concept to get a better understanding of it!
TABLE OF CONTENTS
- Ionic Lattice and Lattice Enthalpy
- Types of Lattice Enthalpy
- Factors Affecting the Lattice Enthalpy
- Comparison between Lattice Energy and Lattice Enthalpy
- Lattice Enthalpy and Born-Haber’s Cycle
- Calculating Lattice Enthalpy from Born-Haber’s Cycle
- Solubility and Lattice Enthalpy
- Practice Problems
- Frequently Asked Questions - FAQ
Ionic Lattice and Lattice Enthalpy
Ionic compounds are generally found in the solid state. An ionic crystal is a highly ordered 3D arrangement of its constituents, cations and anions, which are held together by electrostatic attraction, known as a lattice.
Lattice enthalpy measures the strength of an ionic solid (ionic compound) formed from an ionic bond. The molecules in these ionic solids are organised in a lattice structure, which is a three-dimensional grid. Lattice enthalpy is defined as the strength of the forces between the ions in an ionic solid.
Ionic compounds crystallise in different crystal structures depending upon the size of the ions, their packing arrangements etc.

The stronger the forces, the higher the lattice enthalpy. Lattice enthalpy is the quantity of energy needed to fully separate one mole of the solid ionic compound into its component gaseous ions.
- Energy is required to remove an electron from an atom, whereas energy is released when an electron is added to an atom.
- Therefore, the ease with which the positive and negative ions are produced from the corresponding neutral atoms determines the strength of an ionic combination.
- According to the following descriptions, the ionisation enthalpy and electron affinity determine how easily positive and negative ions form:
- The removal of electrons is necessary for the creation of positive ions.
- Ionisation enthalpy is the amount of energy needed to remove an electron from an isolated gaseous atom.
- Consequently, atoms with lower ionisation potential (ionisation enthalpy) will possess greater lattice energy.
- An electron is gained during the creation of negative ions.
- The amount of energy released when an electron is added to an atom is known as the electron gain enthalpy; hence, the bigger the electron affinity, the greater the lattice energy.
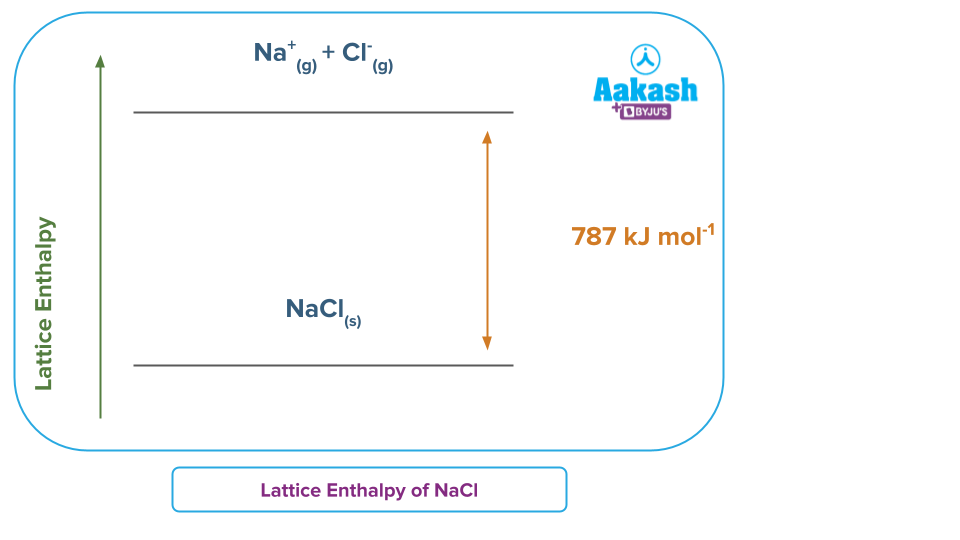
The energy needed for the subsequent reaction to take place in the instance of this ionic molecule is known as the lattice energy.
NaCl(s) → Na+ (g) + Cl- (g)
Here, 1 mole of sodium chloride, requires 786 kJ of energy to be converted into gaseous Na+ and Cl- ions.
Types of Lattice Enthalpy
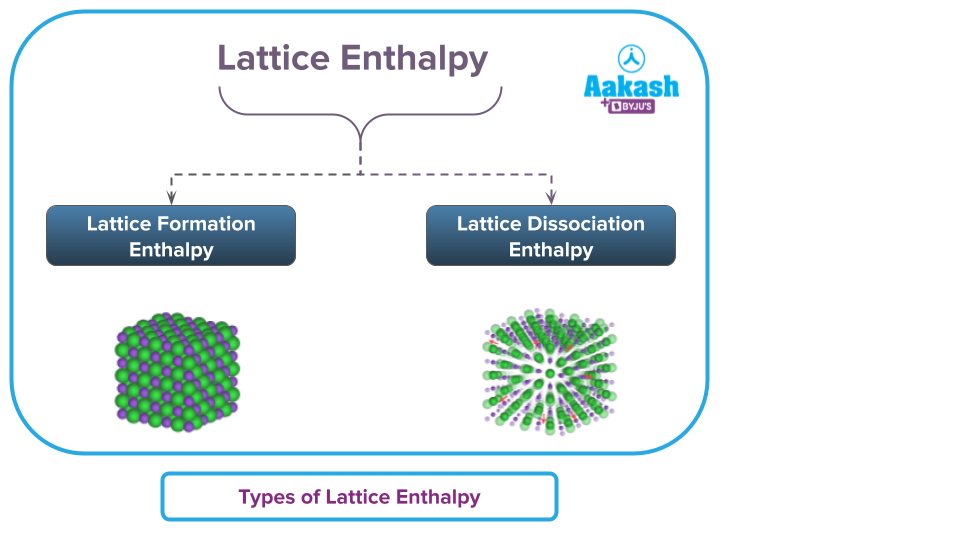
Lattice Formation Enthalpy: The energy released when one mole of an ionic solid compound is completely formed from its constituent ions in the gaseous phase.
A+ (g) + B- (g) --> AB (s)
The enthalpy change that occurs when 1 mole a solid ionic crystal is created from its separated gaseous ions is known as the lattice formation enthalpy. Enthalpies for lattice formation are usually negative.
Thus, lattice formation enthalpy is exothermic as it involves the liberation of heat.
Lattice Dissociation Enthalpy: The energy absorbed when one mole of an ionic solid compound dissociates completely into its constituent ions in the gaseous phase.
AB (s) --> A+ (g) + B- (g)
The enthalpy change required to break down 1 mole of solid crystal into its constituent gaseous ions is known as the lattice dissociation enthalpy. Values of Lattice dissociation enthalpies are positive.
Lattice dissociation and formation enthalpy have the same amount of energy involved. The lattice dissociation enthalpy is endothermic as it involves the absorption of heat.
An ionic compound will be stable if it has a sufficiently negative value of lattice energy.

Factors Affecting the Lattice Enthalpy
- Charge on Ions
Larger the magnitude of charge on the ions, the greater will be the attractive force and consequently, the higher is the value of lattice enthalpy. For example, the lattice enthalpy of magnesium oxide (MgO) is much greater than that of sodium chloride (NaCl).
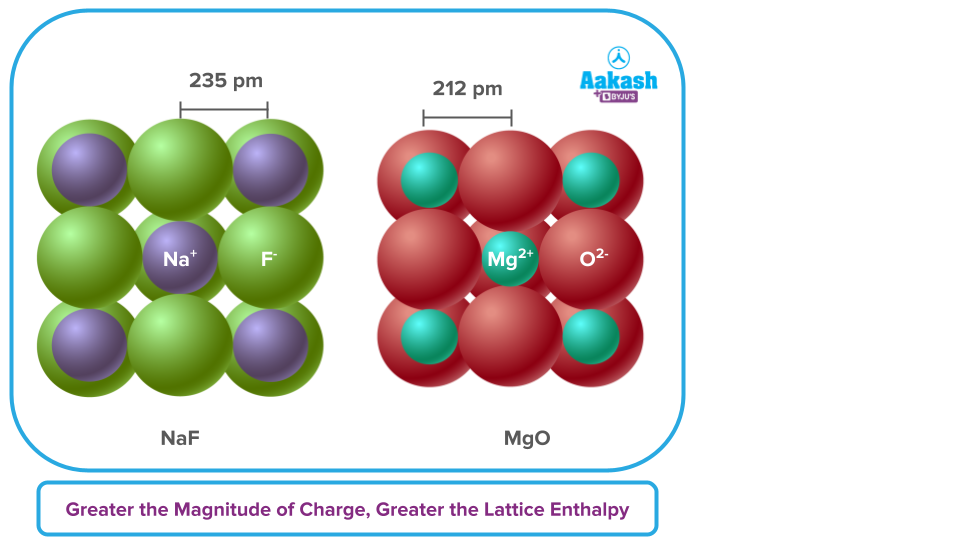
The electrostatic force of attraction that exists between the ions in the lattice crystal draws them together. Since we already know that this force is inversely proportional to the size of the charge, the stronger the lattice, the larger the charge.
Lattice Enthalpy ∝ Z+, Z-
Where, Z+ = Charge on Cation in terms of Electronic Charge
Z- = Charge on Anion in terms of Electronic Charge
For instance, although KCl and CaCl2 have the same crystal structure, the latter has a higher lattice enthalpy. This is because calcium ions have two positive charges, but potassium ions only have one (the strength of the electrostatic force of attraction is directly proportional to the charge).
- Size of Ions/ Ionic Radii
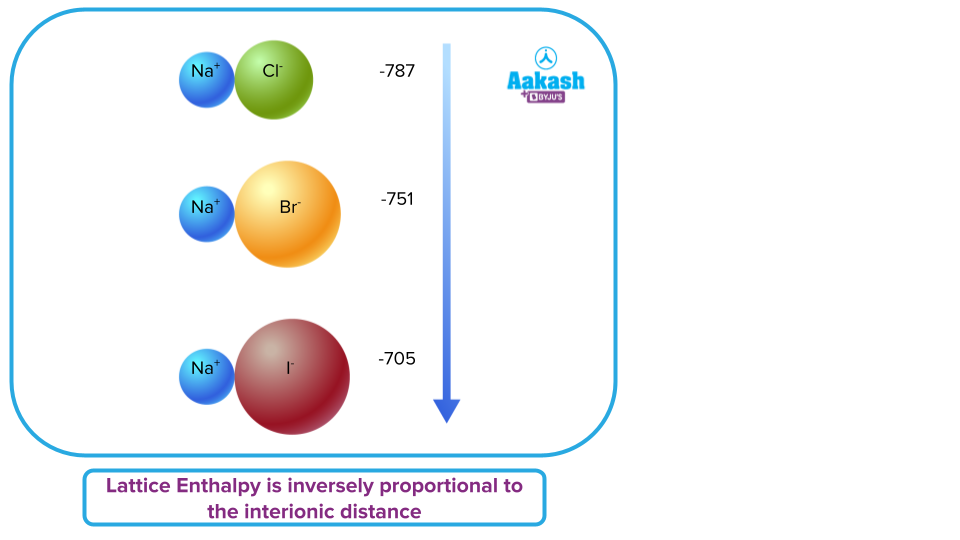
Smaller the size of ions, the lesser the internuclear distance and the greater will be the inter-ionic attraction, hence, more will be the value of lattice enthalpy.
Smaller ions have higher binding forces because they have closer interionic distances. Higher lattice enthalpy is the outcome of this. For instance, the lattice energy of their sodium counterparts decreases as we move from fluoride to iodide in group 16 of the periodic table.
NaF > NaCl > NaBr > NaI
Smaller the ions, the closer they are together in the lattice, and that increases the strength of the attractions.
Lattice energy (L.E.) ∝
r = r+ + r- = Interionic Distance (Ionic Radii)
- Coordination Number
Coordination number is important only in the case of sulphates and carbonates of alkaline earth metals. The coordination number is calculated experimentally by the radius ratio.
L.E. ∝ coordination number (C.N.)
C.N. of cation: No. of anions surrounding the cation.
C.N. of anion: No. of cations surrounding the anion.
Lattice energy order for sulphates of alkaline earth metals is as follows.
BeSO4 < MgSO4 < CaSO4 < SrSO4 < BaSO4
Size of cation
Comparison between Lattice Energy and Lattice Enthalpy
The following equation may be used to represent the molar lattice energy of an ionic crystal in terms of molar lattice enthalpy, pressure, and volume change.
ΔGU = ΔGH – pΔVm
Where, ΔGU = Molar Lattice Energy (Molar Internal Energy Change)
ΔGH = Molar Lattice Enthalpy.
ΔVm = Change in Volume per mole.
p = Pressure.
The external pressure is also taken into consideration while calculating lattice energies of ionic solids. For solids, the molar volume is much smaller than that of gases, so ΔVm < 0. Due to the net attractive forces present, the creation of a crystal lattice from ions in vacuum must reduce the internal energy. So, ΔGU < 0. So, the term – pΔVm is positive but comparatively small at low pressures, therefore the lattice enthalpy of formation of solids is negative and the process is exothermic.
Lattice Enthalpy and Born-Haber’s Cycle
For calculating lattice enthalpy from Born Haber’s Cycle, we need to understand Hess’ law first. According to Hess's law, whether a reaction occurs in a single step or over the course of several stages, the change in enthalpy that results from the conversion of a reactant into a product is always the same. So, to obtain the overall change in enthalpy, we apply constant heat summation, wherein the total heat of the reaction is constant.
Hess's law is used by the Born Haber Cycle to determine lattice enthalpy.
For example, let us represent a chemical reaction as A → B; The heat of the reaction (H= Q).
Let us consider the reaction to have taken place through a series of several steps:
So, applying Hess’ law, Q can be obtained as: Q = Q1 + Q2 + Q3. It is impressive how this law is applicable for cyclic processes too.
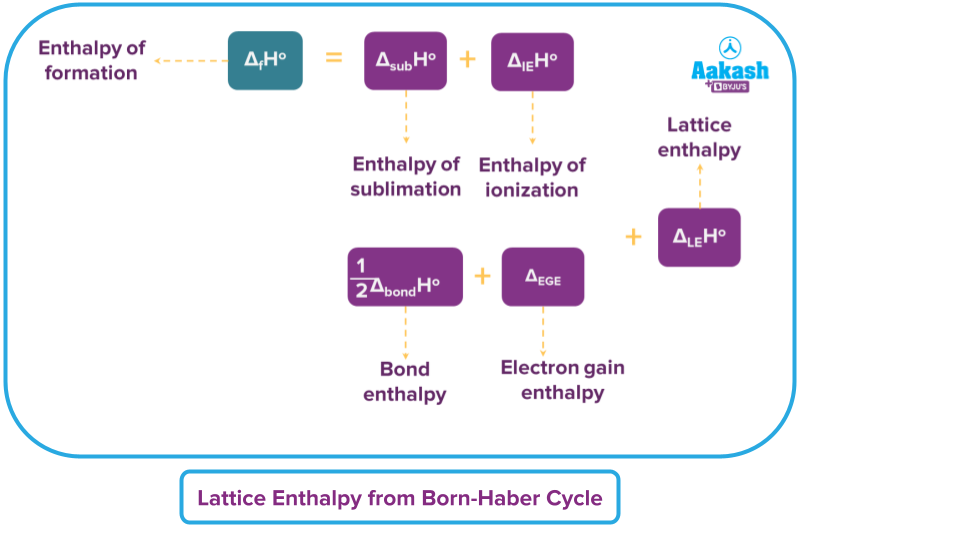
Calculating Lattice Enthalpy from Born-Haber’s Cycle
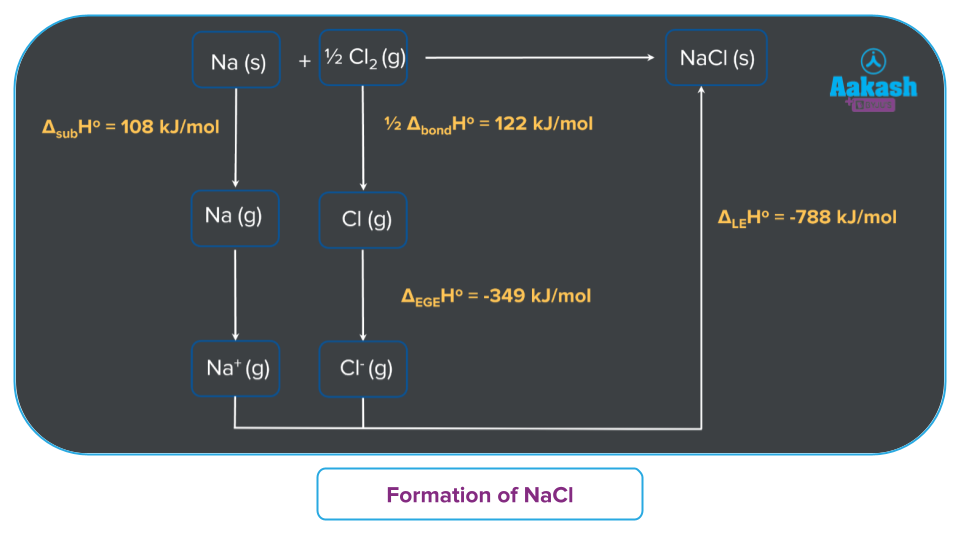
Consider the formation of the ionic solid NaCl.
- First step is the sublimation of sodium atoms from solid to gaseous form. This process needs energy, specifically sublimation energy ().
- The sodium atom loses an electron to become a sodium ion during the ionisation process. The ionisation enthalpy () is the energy supplied during this process.
- The energy needed to cause the chlorine molecule to split into two chlorine atoms is called the dissociation energy () for Cl2.
- Chloride ion (Cl-) formation occurs when the Cl atom accepts an electron. The electron affinity is the energy released ().
- Sodium chloride is created when chloride ions interact with sodium ions to generate sodium chloride. The lattice energy is the total amount of energy released ().
Hence, the enthalpy of formation of NaCl is expressed as:
Solubility and Lattice Enthalpy
The lattice enthalpy of an ionic solid and the hydration energy of the ions are the major factors that determine the solubility of that compound.
- Because of the effect of the higher magnitude of charge on the ions of Group II metals, some lattice enthalpy values for Group II compounds are significantly higher than the values for Group I compounds for the same anion.
- The lattice energy of each individual negative ion reduces as the metal's size rises. As the metal ions get bigger, so does the hydration energy.
- The hydration energy must be greater than the lattice energy for a material to dissolve.
- Higher lattice energies often result in less solubility of a salt, and the salt with the smaller cation has the higher lattice energy.
- To determine solubility, the cation's solvation energy is also crucial, and tiny cations often have greater solvation energies. The solubility of ionic compounds often increases with high solvation energies.
- But a decrease in the energy of hydration favours a decrease in solubility. When a salt is dissolved in water, the outermost ions (those at the lattice's edge) leave the lattice and are covered by the nearby water molecules.
- Therefore, solubility increases if hydration enthalpy is more than or equal to lattice energy, and decreases if it is lower.
Practice Problems
Q1. Give the correct solubility order:
- LiNO3 < NaNO3 < KNO3 <RbNO3 < CsNO3
- NaNO3 < KNO3 < LiNO3 < RbNO3 < CsNO3
- LiNO3 < NaNO3 < CsNO3 < KNO3 < RbNO3
- RbNO3 < LiNO3< KNO3 <NaNO3 < CsNO3
Answer: A
Solution: The anion is the same for all (NO3-). The size of cations of alkali metals increases on going down the group.
As we go down the group, the ionic radii increase, and hence the ionic character increases. Also, the overall decrease in lattice enthalpy is greater as compared to the hydration enthalpy. Hence, the solubility increases down the group.
The solubility order of nitrates is LiNO3 < NaNO3 < KNO3 <RbNO3 < CsNO3
Alternatively, we can also say that NO3- is highly polarised by the smallest sized Li+ cation and hence, it is the most covalent nitrate (LiNO3) and is the least soluble.
So, option A is the correct answer.
Q2. What is the effect of high lattice energy on ionic compounds?
Solution: Since ionic compounds frequently have crystalline lattice structures, they typically have high melting points. Also, high lattice enthalpy leads to compact solid structures which are thermally stable and resistant to heat.
Q3. Lattice Enthalpy of LiF with respect to MgO is:
- Greater
- Lesser
- Same
- Unknown
Answer: B
Solution: The magnitude of charge on the ions of lithium fluoride is +1 (Li) and -1 (F) as opposed to +2 (Mg) and -2 (O) in MgO. Thus, due to the lesser charge on LiF, its lattice enthalpy is lesser than that of MgO.
So, option B is the correct answer.
Q4. Lattice Enthalpy is inversely proportional to
- Ionic Radii
- Charge on Anion
- Charge on Cation
- Covalent Radii
Answer: A
Solution: Lattice enthalpy is inversely proportional to ionic radii. The smaller the ions, the closer they are together in the lattice, which increases the attraction's strength.
Lattice energy (L.E.) ∝
r = r+ + r- = Interionic Distance (Ionic Radii)
So, option A is the correct answer.
Frequently Asked Questions - FAQ
What is the relation between thermal stability and lattice enthalpy?
The greater the lattice enthalpy of an ionic solid, the stronger the electrostatic force of attraction between the constituting ions and hence difficult it is to break the ionic bond. Hence, thermal decomposition becomes difficult in such cases where lattice energy is high.
Is lattice enthalpy present in covalent compounds?
Ideally no, because lattice enthalpy corresponds to the strength of the electrostatic force of attraction between oppositely charged ions in the gaseous state and that is majorly seen in ionic compounds. Nevertheless, some covalent compounds such as sucrose may also form lattice patterns, although the forces that keep them together are weaker than those of ionic compounds.
Lattice energies are linked to a variety of interactions in ionic compounds because cations and anions form an extensive lattice. Whereas for covalent compounds, the interaction of merely two atoms is what causes the bond dissociation energy for covalent bonds.
How does lattice energy and hydration energy contribute to a compound’s solubility?
Both lattice and hydration energy contribute to solubility, although occasionally one term predominates over the other. An additional relation was developed to explain this dominance. The outcome of two factors can be stated as
When a salt is dissolved in water, the outermost ions (those at the lattice's edge) leave the lattice and are covered by the nearby water molecules. Therefore, the salt is water-soluble if the hydration energy is equal to or higher than the lattice energy.
What is the difference between lattice energy and lattice enthalpy?
Although the terms are used alternatively, lattice enthalpy is a unit of measurement for the strength of the forces between the ions in an ionic solid. The lattice enthalpy increases with the strength of the forces. Whereas, lattice energy is the amount of energy needed to split a mole of an ionic solid into gaseous ions.


