-
Call Now
1800-102-2727
Ketones Nomenclature: Introduction, Structure, Nomenclature, Uses of Ketones, Practice Problems & Frequently Asked Questions(FAQs)
Do you know that the tablet we take while sick, contains less than 5% of the active component? The rest are called fillers that are inactive and added to add volume. But, these active and inactive materials are transferred into a uniform mixture by dispersing them in a liquid (solvent) medium. The solvent chemical used is acetone which belongs to the ketone family.

Because it helps deliver the right quantity of active compounds and fillers, acetone is frequently employed as an excipient in regularly prescribed drugs. One of the most common applications for acetone as a solvent is in the manufacturing of pharmaceuticals. The majority of tablets will be tough to compress into an appropriate density and won't dissolve entirely if acetone isn't included in the medication
It is non-toxic and can be absorbed by the skin, inhalation, or both. Its chemical reaction causes an increase in the synthesis of glucose in living cells. It has a depressive effect on the central nervous system when concentration is high.
Actually, Joseph Lister used acetone as an antiseptic for the first time.
Table of Contents:
- Structure of Ketones
- IUPAC Nomenclature of Ketones
- Uses of Ketones
- Practice Problems
- Frequently Asked Questions(FAQs)
Structure of Ketones:
Ketones are organic compounds containing a keto functional group. A keto functional group is a carbonyl group attached on either side of the carbonyl carbon by the same or different alkyl or aryl or a mixture of alkyl and aryl groups.
Organic ketones have the functional group -C=O and the structural formula R (C=O) R'. In this instance, R and R' may be aryl or alkyl groups. A sp2 hybrid orbital surrounds the carbonyl carbon of the ketone group. A triangular planar structure can be observed in the ketone. The estimated bond angle of this structure is 120o. Due to the polarization of carbonyl groups by carbon-oxygen bonds, ketones have an oxygen atom that is nucleophilic and a carbon atom that is electrophilic.(oxygen is stronger electron-withdrawing than carbon).

We will look at the first member of the ketone homologous series, commonly known as Acetone.
Acetone is also known as propanone by IUPAC nomenclature and has the chemical formula C3H6O. Six hydrogen atoms, three carbon atoms, and one oxygen atom make up acetone. It is classified as a ketone since it has a (-C=O) carbonyl group. It is a dimethyl ketone derived from propane that has an oxo group connected to the carbon atom in the second position. The chemical formula for acetone is
(CH3COCH3).
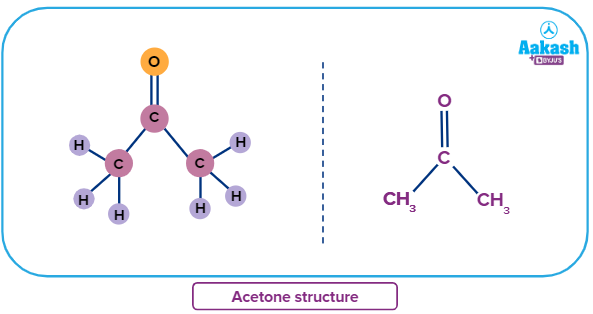
A symmetrical ketone is one whose two groups on the carbonyl carbon are similar in the molecule.
Examples: Acetone and Benzophenone.


An unsymmetrical ketone is one whose two hydrocarbon groups on the carbonyl carbon are different in the molecule.
Example: Acetophenone.

IUPAC Nomenclature of Ketones:
Atoms or groups other than hydrogens attached to carbon in a hydrocarbon are considered either substituents or functional groups in the IUPAC nomenclature of organic compounds. These atoms or groups of atoms regulate the properties and unique chemical reactions of an organic molecule. They are included as either a suffix or a prefix to the parent hydrocarbon nomenclature as follows.
The IUPAC Naming system of organic compounds has a fundamental skeleton as-
Secondary prefix + Primary prefix + Root word + Primary suffix + Secondary suffix
Where,
Secondary prefix - specifies all substituents except that of the suffix functional group attached to the parent chain
Primary prefix - specifies the nature of the parent chain (cyclo, bicyclo, spiro)
Root Word - specifies the number of carbon atoms in the parent chain
Primary Suffix - specifies the saturation of the parent chain
Secondary Suffix - specifies the functional group.
In general, in the IUPAC nomenclature, functional groups are included as a suffix or a prefix to the parent hydrocarbon from which they may be considered derived. The functional group(s)-
- The carboxylic acid functional group always goes a suffix
- Halo, nitro, alkyl or aryl substituents are always placed as prefixes and
- All other functional groups as
i) suffix if they are either the only functional group or if they are the most preferred among the
multiple functional groups present in the compounds and
ii) prefix if there is another most preferred functional group in the compound.
In the IUPAC nomenclature system of organic compounds, the keto functional group may be included as either a suffix or prefix.
Keto functional group in suffix:
When ketones are either the only functional group or the priority functional group present in the compound, then they are indicated in the secondary suffix as ‘one’
It should be well remembered that the ketonic carbon is also to be included in the hydrocarbon longest chain while identifying the parent hydrocarbon. The numbering of the positions of the substituents follows the same IUPAC nomenclature of the minimum rule.
The IUPAC (International Union of Pure and Applied Chemistry) system provides the ketonic compounds with a distinguishing suffix. The parent hydrocarbon chain's "-ane" suffix is replaced by "one,"
According to some fundamental IUPAC guidelines for identifying ketones:
1. The parent chain always contains the ketone functional group. So, the parent compound is the carbon molecule with the longest carbon chain that also has a ketonic group.
2. It is required to include the number showing the position of the ketone group in the name of the parent hydrocarbon since the ‘-ane’ suffix of the parent hydrocarbon's alkane chain is substituted by ‘-one’.
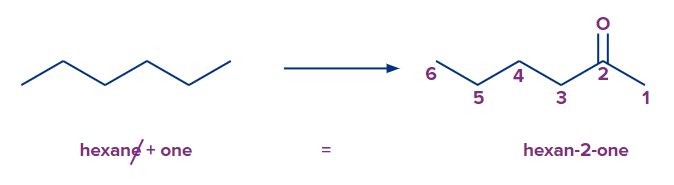
3. Since the ketone functional group is always present in the hydrocarbon chain, it is numbered to have the lowest locant.
4. According to IUPAC standards, the locant identifying the position of the carbonyl group should be mentioned and can be positioned either before the parent or before the suffix "one". For example, butan-2-one or 2-butanone might be appropriate names.
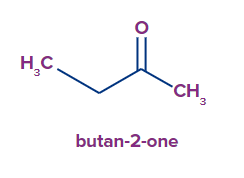
5. The priority of the ketone functional group is less than that of aldehydes. The suffix only changes to "one" if the ketone actually has the highest priority within the molecule.
6. The parent chain is numbered in substituted ketones so that the substituents have the lowest number at the first point of difference. The -OH group works as a substituent in compounds containing ketone and alcohol functional groups (low priority than ketones). As per the functional group's priority order, it is referred to as a hydroxy substituent.
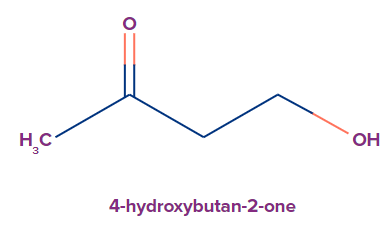
7. The carbon atom of the ring connected to the carbonyl group is assigned the #1 location number when identifying a cyclic ketone.
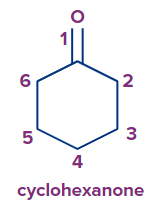
8. The numbering begins at the carbon linked to the ketone group if substituents are also present. It rotates either clockwise or counterclockwise to reduce the amount of substituents
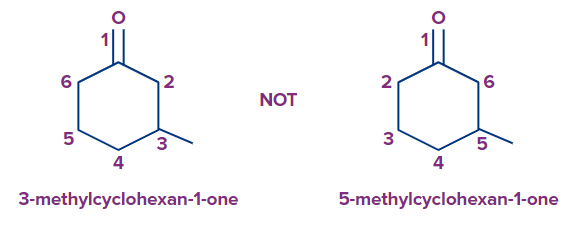
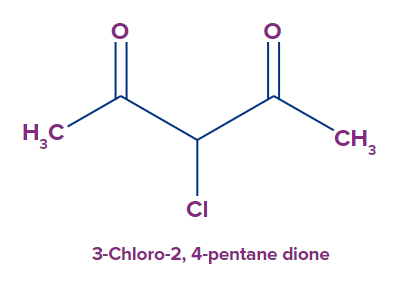
9. For diketones, it is required to give the position numbers for both ketone groups. At the end of the parent chain, the suffix -dione is introduced.
Keto functional group in prefix:
The parent chain of compounds containing a higher priority functional group say carboxylic acid, along with the ketone group will have the suffix of the priority group only and in this case as "-oic acid," The keto group in such case will be treated as a substituent and will be placed as a prefix and remember that the prefix is not ketone but ‘oxo’.
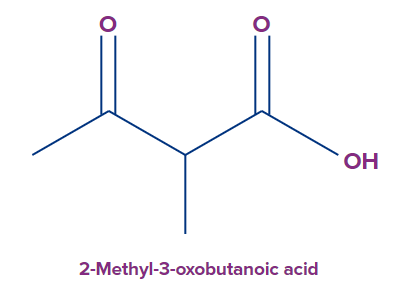
The parent chain is numbered to place the suffix carboxylic group's locant at the lowest.
For example, in the given compound, the numbering starts with the carboxylic acid carbon, and the methyl and the keto groups are substituents at positions 2 and 3 respectively, giving the PUPAC name as 2-methyl-3-oxobutanoic acid.
In the normal naming, identical to ethers, the common names for ketones are generated by identifying each alkyl or aryl group attached to the carbonyl atom as a separate word and adding "ketone" at the end.
The nomenclature of some of the ketones under IUPAC and normal system are given in the table.
|
Formula |
Common name |
IUPAC name |
|
CH3(CO)CH3 |
Acetone |
Propan-2-one |
|
CH3(CO)CH2CH3 |
Ethyl Methyl Ketone |
Butan-2-one |
|
CH3CH2(CO)CH2CH3 |
Diethyl ketone |
Pentan-3-one |
|
CH3CH(CH3)(CO)CH3 |
Isopropyl methyl ketone |
3-Methylbutan-2-one |
|
C6H5(CO)CH3 |
Methyl Phenyl Ketone |
Acetophenone |
Uses of Ketones:
- For several types of plastics and synthetic fibres, ketone performs as a superb solvent. Additionally, it is employed medically to cure acne and perform chemical peeling procedures.
- One of the most often used solvents is butanone, also referred to as methyl ethyl ketone. It is employed in the manufacture of textiles, varnish, paint removers and plastics, among other things. The manufacturing of nylon depends heavily on cyclohexanone, another significant ketone.
- Acetone can be used to erase practically anything, including coffee stains and permanent marker stains, that would otherwise be challenging to wash with water. Acetone can effectively remove stubborn stains from almost any surface because of its outstanding solvent properties. In fact, acetone is so good at cleaning that it is regularly used in labs to clear oil and other lingering pollutants out of beakers and other glassware.
- A wide variety of different cosmetic products are made with acetone. commonly used as a denaturant and solvent in the cosmetics sector. Acetone is a typical chemical found in the manufacture of everything from disinfectant wipes to hair dyes.
- In modern society, fingernails and toenails are routinely painted to enhance their appearance. Most nail polishes are constructed of a mix of polymers, which frequently degrade through a time when subjected to other things. Nail paint remover comes in handy when someone wants to test out a new colour or when their current polish starts to fade. Nail polish remover's main component is acetone.
- When applying nail polish remover, acetone may have an odour that is colourless and unpleasant. Because of its composition and molecular structure, acetone is important in chemistry. In acetone, lone oxygen atom pairs have the capacity to draw hydrogen atoms and form hydrogen bonds. This property makes acetone the favoured solvent in the industry. Because it can dissolve both polar and nonpolar dyes during analysis, acetone, for instance, is preferable to water in chromatography
Practice Problems:
Q1. What is the following compound's IUPAC name?
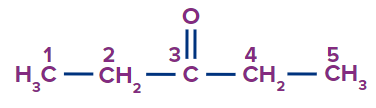
Solution: The given compound is a carbonyl compound known as ketone.
Word root: There are four carbon atoms in the parent chain. So, ‘but’ will be the word root.
Prefix: The parent carbon chain has no substituents connected to it.
Primary Suffix: Since the compound already has a single carbon-carbon covalent bond, and will be employed as the primary suffix.
Secondary Suffix: A secondary suffix of -one will be used because the compound has ketone as a functional group.
Hence, the compound’s IUPAC name is Pentan-3-one or 3- Pentanone.
Q2. What is the following compound’s IUPAC name?
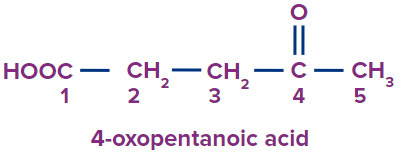
Solution: The given compound has two groups one is a carboxylic acid and another one is a ketone and carboxylic acid is the priority group.
Word root: There are five carbon atoms in the parent chain. So, pent will be the word root.
Prefix: There is an oxo substituent at the fourth carbon atom connected to the parent chain.
Primary Suffix: Since the compound already has a single carbon-carbon covalent bond, and will be employed as the primary suffix.
Secondary Suffix: A secondary suffix of -oic will be used because the compound has carboxylic acid as a priority functional group.
Hence, the compound’s IUPAC name is 4-oxopentanoic acid.
Q3. Why does acetone evaporate more rapidly than ethanol?
(A) Because of hydrogen bonding
(B) Lack of hydrogen bonding
(C) Both A and B
(D) None of the above
Answer: (B)
Solution: Since acetone is a ketone and doesn't have any direct O-H bonds, it doesn't have hydrogen bonding. Despite being alcohol, ethanol possesses a direct O-H interaction. As a result, intermolecular hydrogen bonds exist in ethanol. Because of this, ethanol needs stronger physical bonds to be broken than acetone does. Even though acetone has a higher surface tension than ethanol, it evaporates more rapidly.
Q4. What is the common name of Benzophenone?
(A) Ethyl Methyl ketone
(B) Diphenyl ketone
(C) Methyl phenyl ketone
(D) Diethyl ketone
Answer: (B)
Solution: Diphenyl ketone is the common name for Benzophenone, which has the chemical formula C6H5(CO)C6H5.
Frequently Asked Questions(FAQs):
Q1. Describe diabetic ketoacidosis?
Answer: A serious and life-threatening consequence of diabetes is diabetic ketoacidosis (DKA). Diabetes-related ketoacidosis happens when our body's cells are deprived of the glucose (sugar) they require for energy. This occurs when there is an abundance of glucose in the bloodstream but not enough insulin to aid in the conversion of glucose for cellular usage. When the body realises this, it begins to burn fat and muscle for energy. This breakdown results in ketones (also known as fatty acids), which disrupt our electrolyte balance and induce ketoacidosis (a metabolic acidosis). Because there is insufficient insulin, the sugar that cannot be utilised remains in the bloodstream (rather than going into the cell and providing energy).
Q2. Why does acetone dissolve in both polar and non-polar solvents?
Answer: Oil is a nonpolar solvent, while water is a polar one. But acetone can be dissolved by both of these solvents. Looking at the chemical formula of acetone, we can see that it has two methyl groups. These two methyl groups interact with the oil through dispersion forces since the oil is nonpolar and both of them are methyl groups. Water can form a hydrogen bond with the carbonyl group since it has a polar character and is present. It can also participate in dipole-dipole interactions with other molecules.
Q3. What causes ketones to be acidic?
Answer: Yes, Ketones are those substances that contain a carbonyl carbon joined to two alkyl or aryl groups. The alkyl or aryl group's connected hydrogen atoms are acidic. Ketones have an acidic character because of the acidic hydrogen they contain. However, ketone's acidity is not as high as that of other acids (PKa of the ketone is 17 to 21).
Q4. What applications does acetone have in the laboratory?
Answer: Acetone functions as a polar aprotic solvent for SN2 reactions and other organic reactions carried out in the lab. For the Jones oxidation process to occur, acetone must be used. When dissolved in water, it has a distinct boiling point because it does not mix to form an azeotrope. Due to its low cost and volatility in addition to these features, it is commonly employed as a washing agent in laboratory glassware. Acetone may be cooled using dry ice to as low as 351 K, making it useful for conducting reactions at reduced temperatures. In studies involving fluid movement, the vapour of acetone functions admirably as a fluorescent tracer because it glows under UV light. Occasionally, acetone is used to precipitate proteins.


