-
Call Now
1800-102-2727
Isobaric Process - Definition, Thermodynamic Changes, P vs V Plot, T vs V Plot, Work Done, Practice Problems and FAQs
On one burner, rice is going to be boiled in water in a pressure cooker, while on the other, rice is going to be boiled in an open vessel.

We know in an isothermal process, the temperature remains constant throughout. But what about the “process” of cooking the rice? What kind of thermodynamics processes would that be?
Of course, it can’t be isothermal. The whole idea of cooking is that we raise the temperature to break down complex molecules in food so that it becomes easier for us to digest. So, definitely, both these processes are not isothermal.
Now, before we go into discussing the two processes of cooking rice. Let’s first define our two systems here. The water and rice are in an open vessel in one system and the rice and water are inside the pressure cooker in another.
Now that we have defined the systems, let’s look at the state of the two systems. Before starting to cook them. Let’s assume that both the systems were at room temperature and open to the atmosphere, so the pressure was 1 atm initially. Both the systems are now on the burners and we started heating those systems.
From here on, things would change. We have closed the pressure cooker using its lid and turned on both the gas flames. This means their states are going to change due to the heat supplied. We can see the temperature going up already. The pressure will be increased in the system where rice is going to be cooked in the pressure cooker, so this process can’t be isobaric.
Now, look at this open vessel first. Since it is directly in contact with the atmosphere, the pressure over the system will be equal to atmospheric pressure throughout the process, right? So this is a process where pressure remains the same throughout and hence is called an isobaric process.
Let’s understand more about the isobaric process.
Table of Content
- Definition of Isobaric Process
- Thermodynamic Changes During an Isobaric Process
- P vs V Plot for Isobaric Process
- T vs V Plot for Isobaric Process
- Work Done in an Isobaric Process
- Practice Problems
- Frequently Asked Questions-FAQs
Definition of an Isobaric Process
It is a thermodynamic process in which the pressure will remain constant throughout the process.
Thermodynamic Changes During an Isobaric Process
An illustration is given in the figure below to understand the thermodynamic changes happening during an isobaric process. Consider the process at a micro level, where the gas particles start moving with a greater speed on increasing the temperature as their kinetic energy increases. Further, this increases the collision frequency of the gas particles. Therefore, with the increase in the temperature the volume increases and the piston goes up.
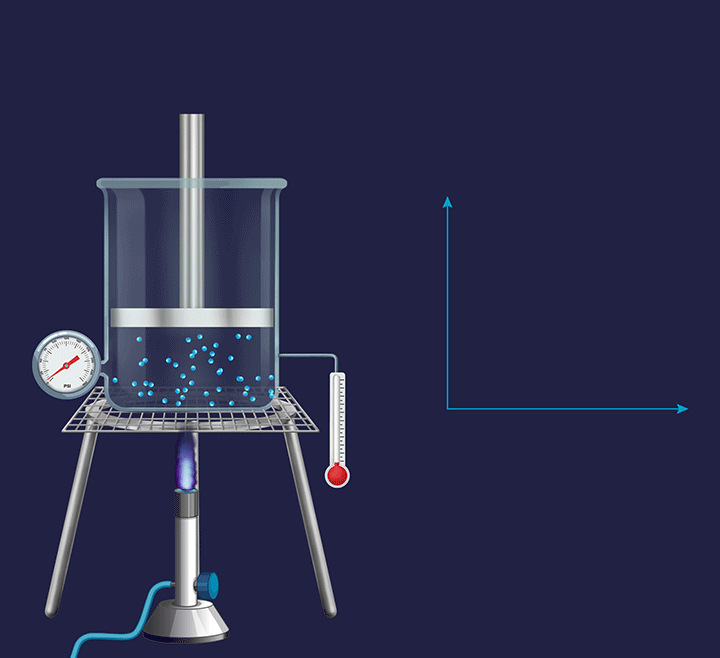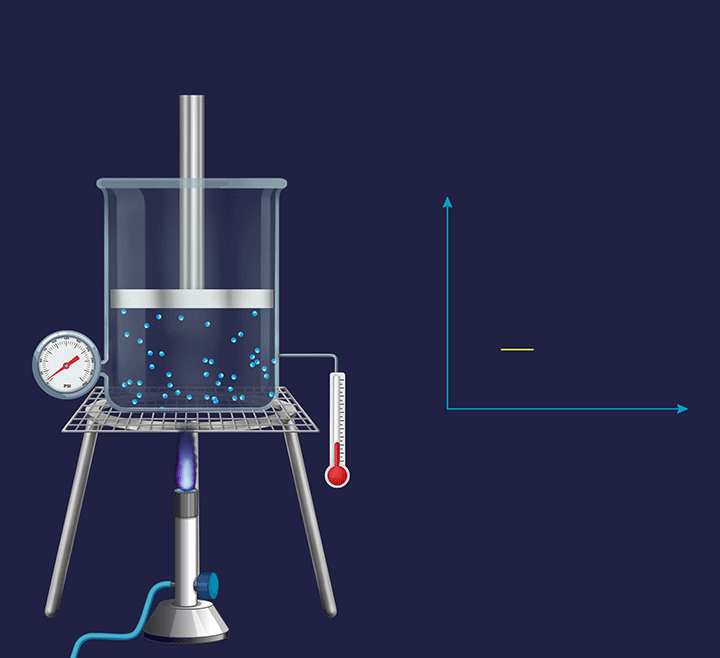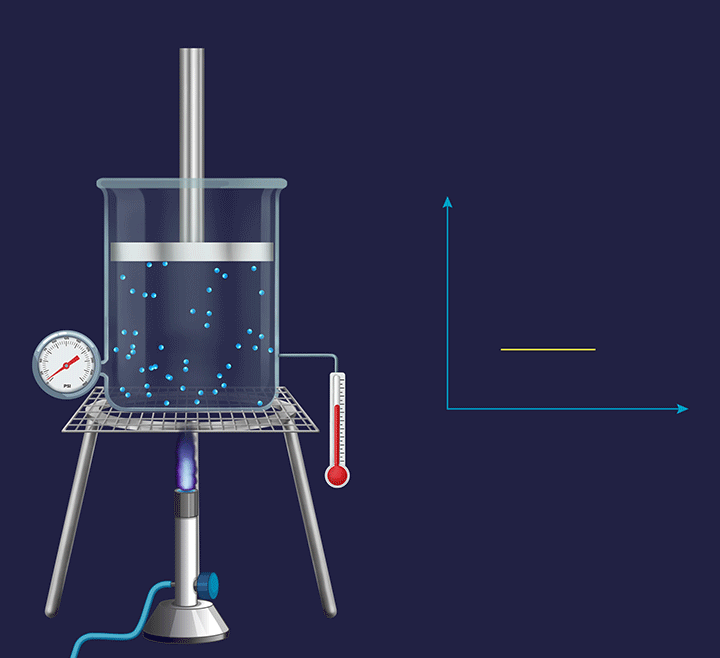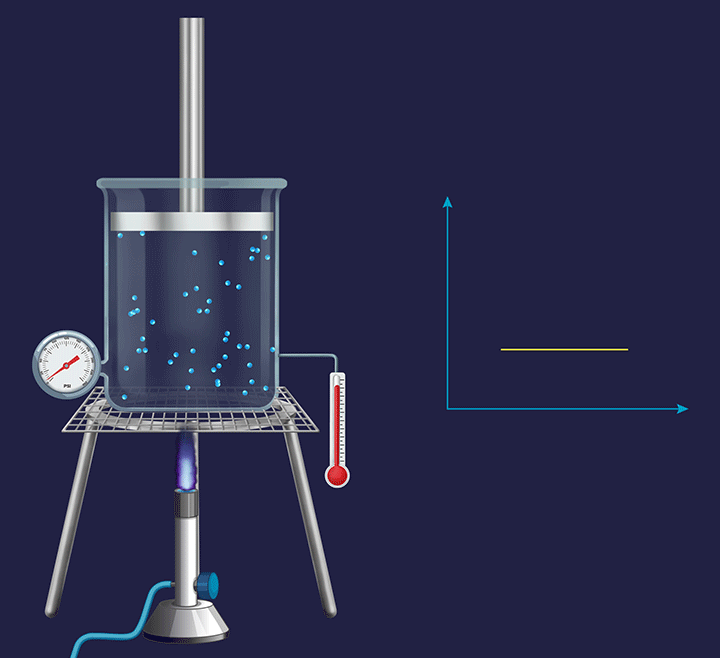
Now, if the process is seen at a macro level, then the pressure exerted by the gas molecules (Pgas) virtually remains constant. However, overall, the volume of a gas increases with the increase in temperature. When the overall expansion of gas is divided into infinitesimal (i.e., very small) expansions, the piston remains stationary after each of these expansions, indicating that the external atmospheric pressure (Patm) is balanced by the internal pressure exerted by the gas particles (Pgas).
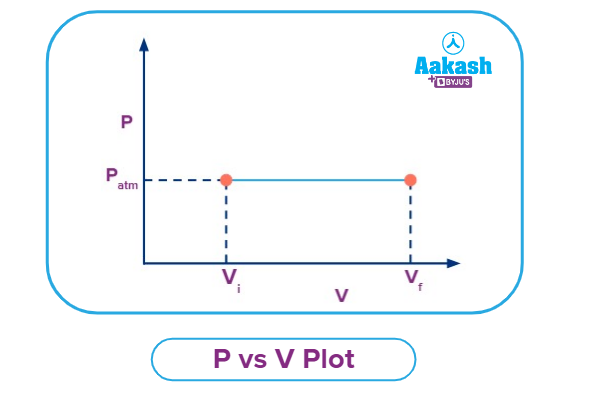
P vs V Plot for an Isobaric Process
The pressure vs volume plot for an isobaric process is given in the figure below. On increasing the temperature, the gas is expanding from Vi to Vf by maintaining a constant pressure as shown in the graph. However, on decreasing the temperature, the gas will compress by maintaining a constant pressure.
Patm
vi
vf
V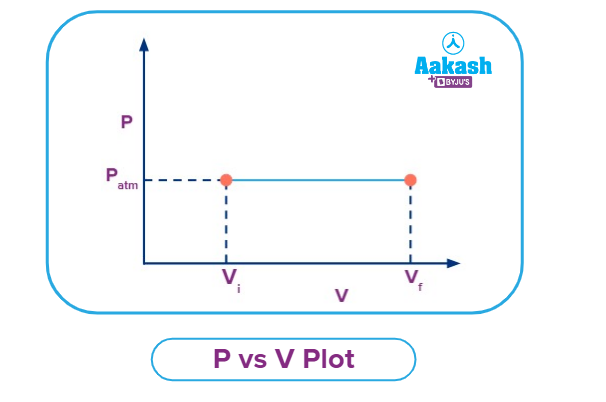
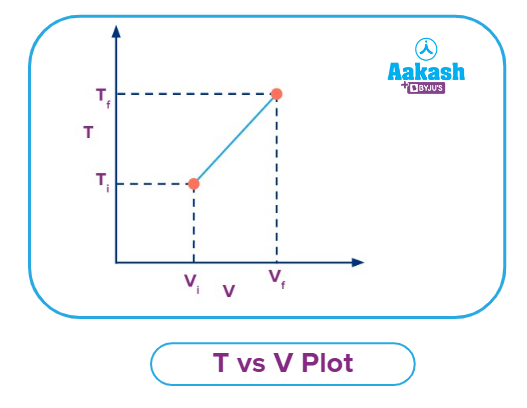
T vs V plot for an Isobaric Process
It is observed that the volume occupied by the gas particles increases with the increase in temperature, i.e., the temperature is directly proportional to the volume at constant pressure and for the fixed amount of the gas.
Consider an ideal gas;
PV=nRT
As P, n & R being the constants.
⇒ T ∝ V
So the curve of T vs V will be a straight line and if extrapolated it will pass through the origin.
Work Done in an Isobaric Process
Work done can be calculated using the formula given below:
From the illustration which is discussed, it was observed that for the piston to remain stationary, the external atmospheric pressure is to be balanced by the pressure exerted by the gas particles and thus, maintaining a constant pressure, i.e., Pext=Pgas
Thus, the equation (i) becomes;
Integrating from initial volume (Vi) to final volume (Vf), we get;
So,
This formula of work done is true for both reversible and irreversible isobaric processes.
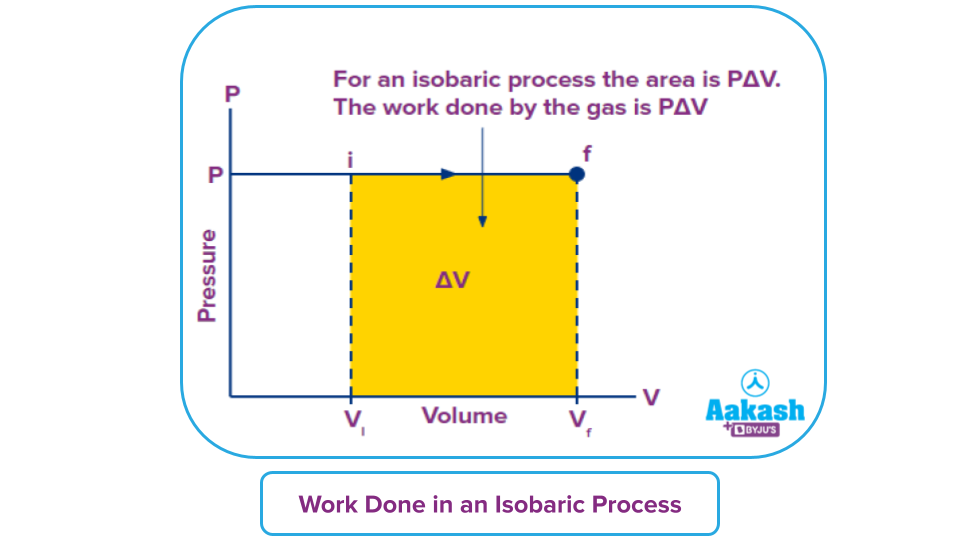
Calculation of work using the P-V graph:
Work done on the gas is basically the area under the P-V curve. As pressure remains constant in the isobaric process, so area will be the product of constant pressure and change in volume as shown below.
Practice Problems
Q. Which of the following is not an example of an isobaric process?
- Boiling of water
- Freezing of water
- Boiling of water in a pressure cooker
- Both A and B
Answer: (C)
Solution: The process of converting water into steam or ice is an illustration of the isobaric reaction. A gas either expands or contracts during the process to maintain constant pressure. Both boiling of water and freezing of water are examples of isobaric processes. The boiling of water in a pressure cooker is not an example of the isobaric process as pressure can’t remain constant during the process of boiling.
Q. In which process the P-V diagram is a straight line parallel to the volume axis?
- Isothermal
- Isochoric
- Isobaric
- None
Answer: (C)
Solution: The pressure vs volume plot for an isobaric process is given in the figure below. On increasing the temperature, the gas is expanding from Vi to Vf by maintaining a constant pressure as shown in the graph.
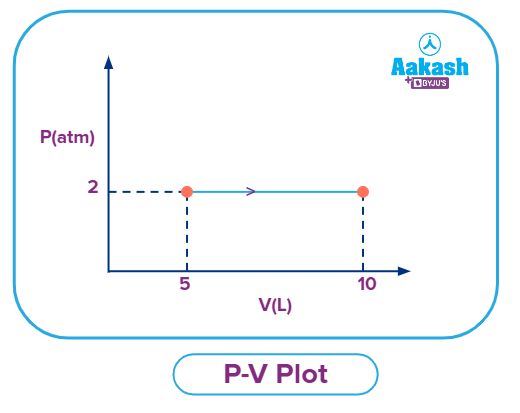
Q. A gas expands at a constant pressure of 5 atm from 5 L to 10 L. The work done by the gas is:
- - 25 atm L
- 25 atm L
- - 5 atm L
- 5 atm L
Answer: (A)
Solution: As the process is occurring at constant pressure, hence it is an isobaric process and the formula for calculating the work done in an isobaric process is:
Now,
So,
Q. A gas expands at constant pressure as shown below. The work done by the gas is:
- - 10 atm L
- 10 atm L
- - 15 atm L
- - 5 atm L
Answer: (A)
Solution: Work done by the gas is the area under the P-V curve.
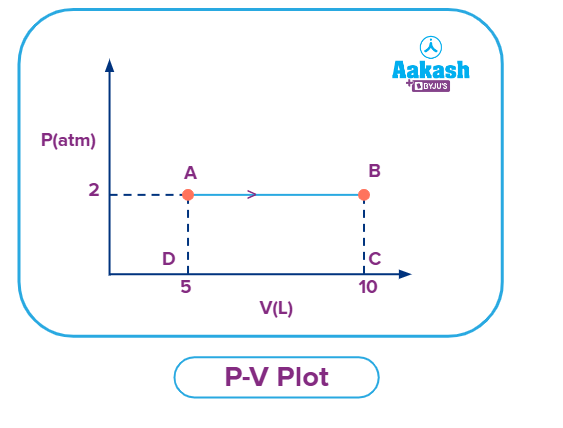
Now, Work Done = Area of rectangle ABCD = (AD × AB) = (2 × 5) = 10 atm L
As the gas is expanding, the work done will be negative as work is done by the gas which is -10 atm L .
Frequently Asked Questions-FAQs
Q. What conditions are needed for a process to be isobaric?
Answer: A thermodynamic process known as an isobaric process occurs under constant pressure. Temperature, volume, and internal energy are not constant in this process, even though the pressure is constant.
Q. Is there any heat transfer that occurs during the isobaric process?
Answer: Heat enters the system during an isobaric expansion process and heat is given out by the system during the isobaric compression process.
Q. Can a process that is isobaric be reversed?
Answer: Processes that take place under constant pressure are called isobaric processes. So, certainly, we may perform an isobaric process slowly and reversibly if we are able to regulate the change in other macroscopic characteristics of the system so that they change slowly enough.
Q. Is freezing isobaric in nature?
Answer: Since the phase change takes place at a constant temperature, freezing is an isothermal process. A thermodynamic process in which the pressure stays steady is an isobaric process. But we can say that freezing is also an isobaric process because there is no pressure change that occurs during the process.


