-
Call Now
1800-102-2727
Ionisation Energy – Definition, General Trends, Applications, Practice problems, FAQs
Watching auroras is a delight. When the sun's extremely energetic particles interact with the gases in the upper atmosphere, they are created. The upper atmosphere's gaseous atoms exchange electrons when they come into contact. Regaining the electrons that were stolen from the atoms causes radiation to be released, which is visible in the sky as a variety of coloured clouds.
The atoms are ionised—that is, they are changed into ions—during this process. Ionization is the process of adding or withdrawing an electron from a neutral atom to change it into an ion. The elimination of electrons and the energy involved in this process will be the main topics of discussion on this concept page.

Moving forward, it's important to keep in mind that, depending on the situation, this ability can also be demonstrated by other elements in the periodic table in addition to those that exist as gases.
In order to remove an electron from an atom, we must exert some energy against the nuclear attraction that pulls electrons toward the nucleus. These valence electrons are the furthest from the hold of nuclear charge, making it easier to remove them. However, depending on the strength of the outermost electron's nuclear attraction, this ease of electron removal differs from atom to atom. Others are challenging to ionise because of the strong nuclear attraction, however some elements are easy to ionise, or we can say they have a greater tendency to generate ions. Ionisation energy, also referred to as ionisation energy, is a method used to quantitatively assess an element's propensity to lose electrons.
Let's learn more about ionisation energy, the factors that influence it, the trend of ionisation energy in the periodic table, and its uses right away.
TABLE OF CONTENTS
- What is Ionisation Energy?
- General Trends in Ionisation Energy across the Periodic Table
- The First Ionisation Energy of the First 60 Elements in the Periodic Table
- Successive Ionisation Energies
- Factors Affecting Ionisation Energy
- Metallic Character and its Relation with Ionisation Energy
- Applications of Ionisation Energy
- Practice Problems
- Frequently Asked Questions - FAQs
What is Ionisation Energy?
The minimum energy required to remove the most loosely bound electron from an isolated gaseous atom in its ground state is called ionisation energy.
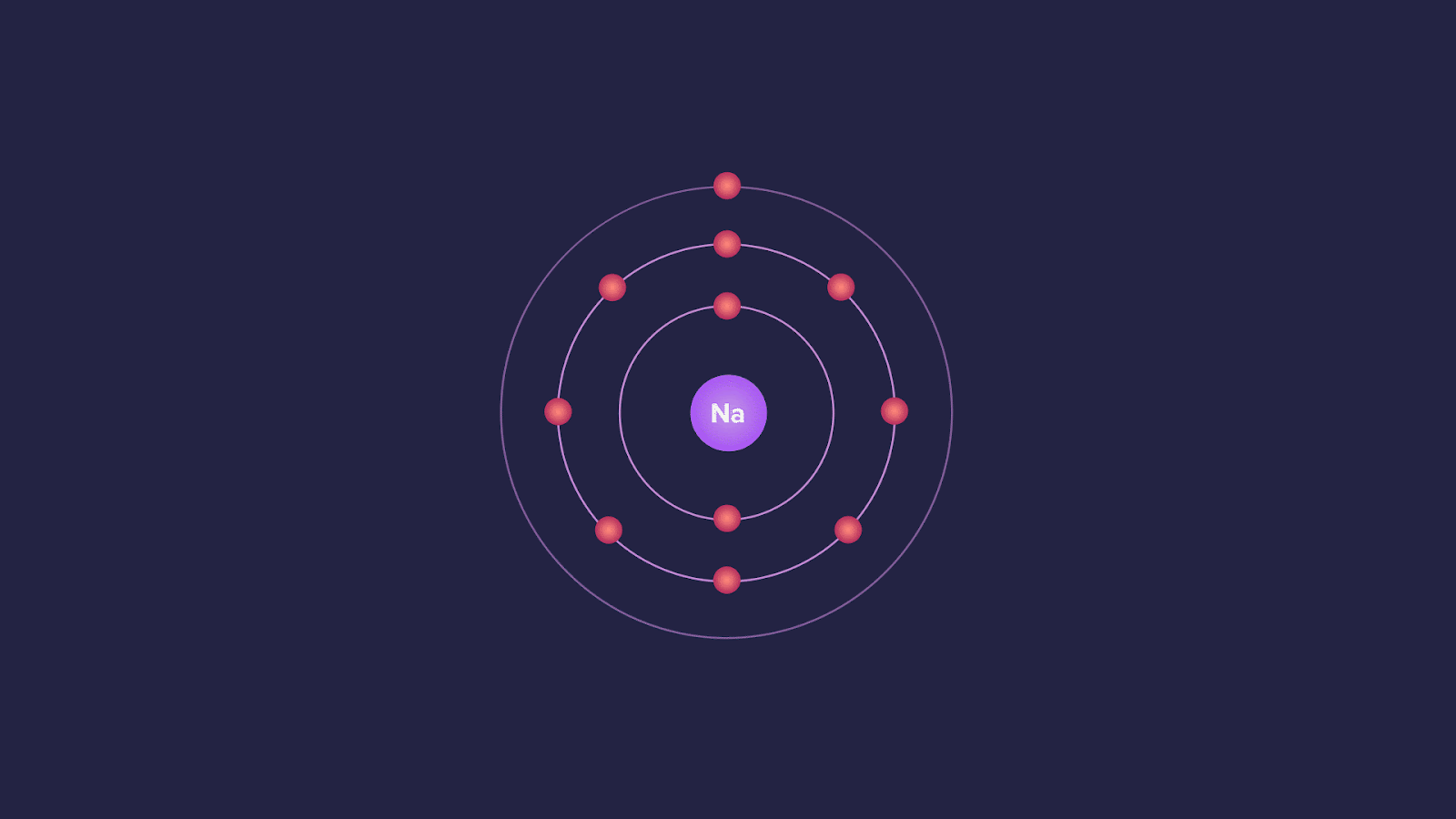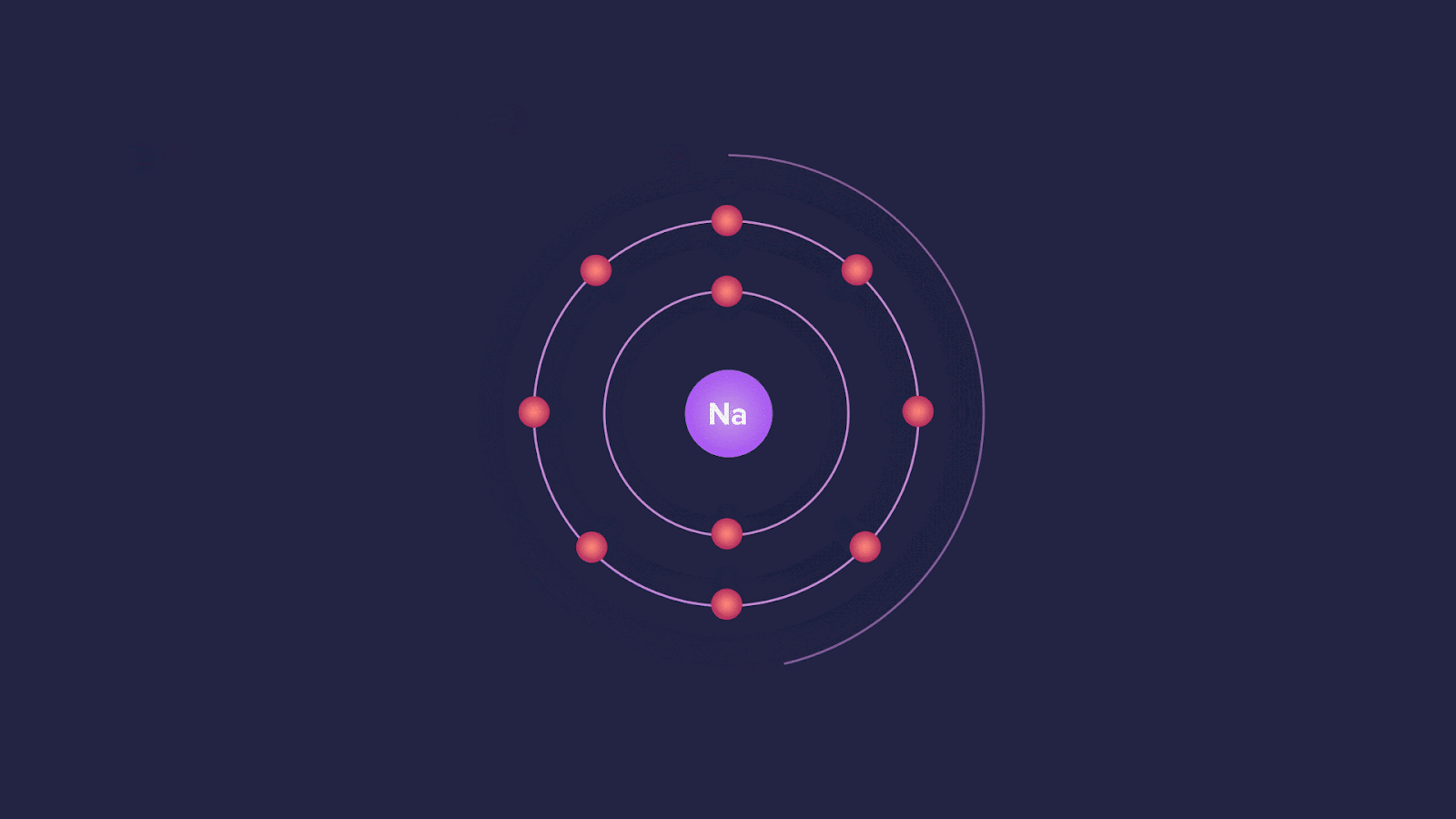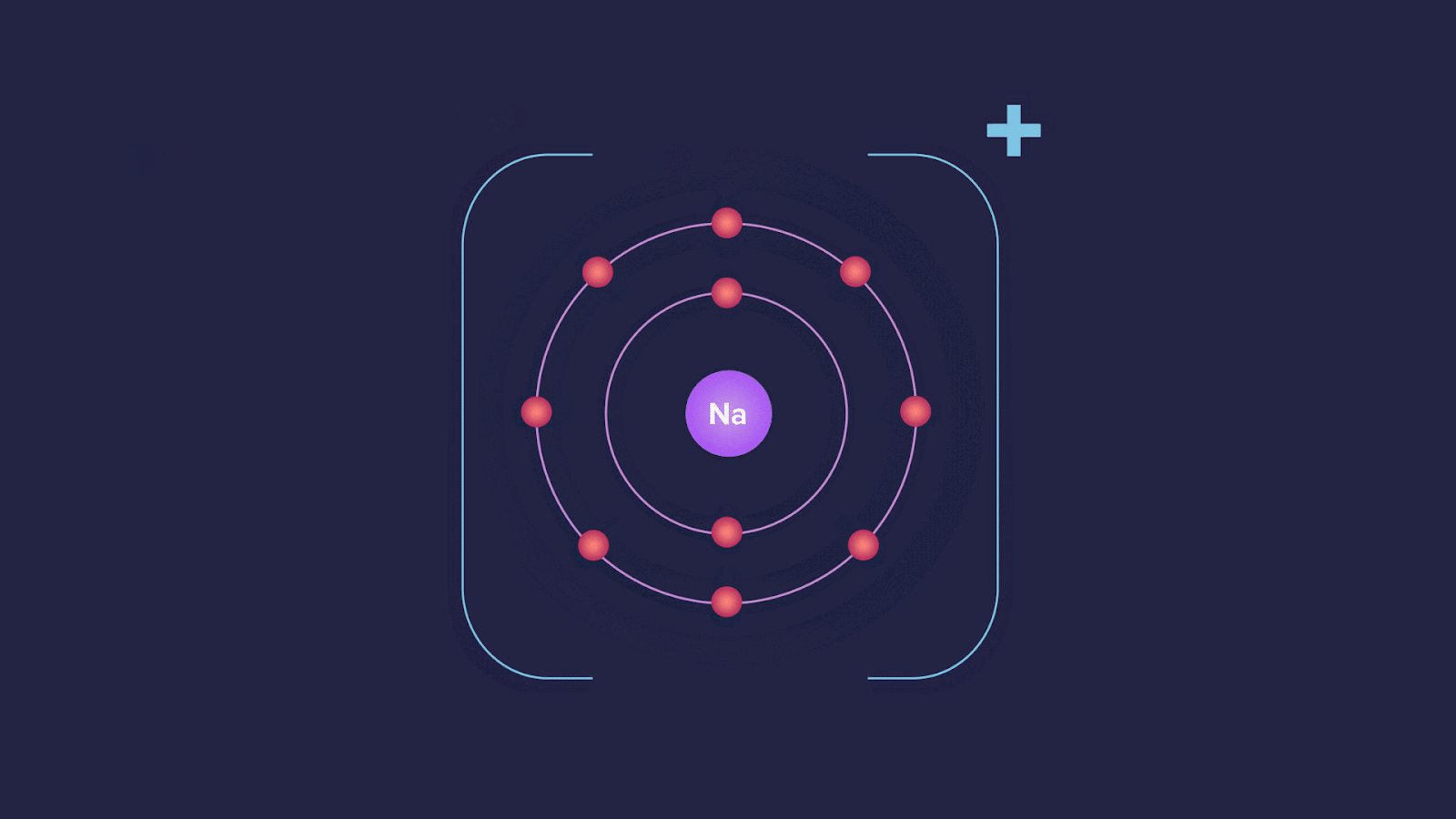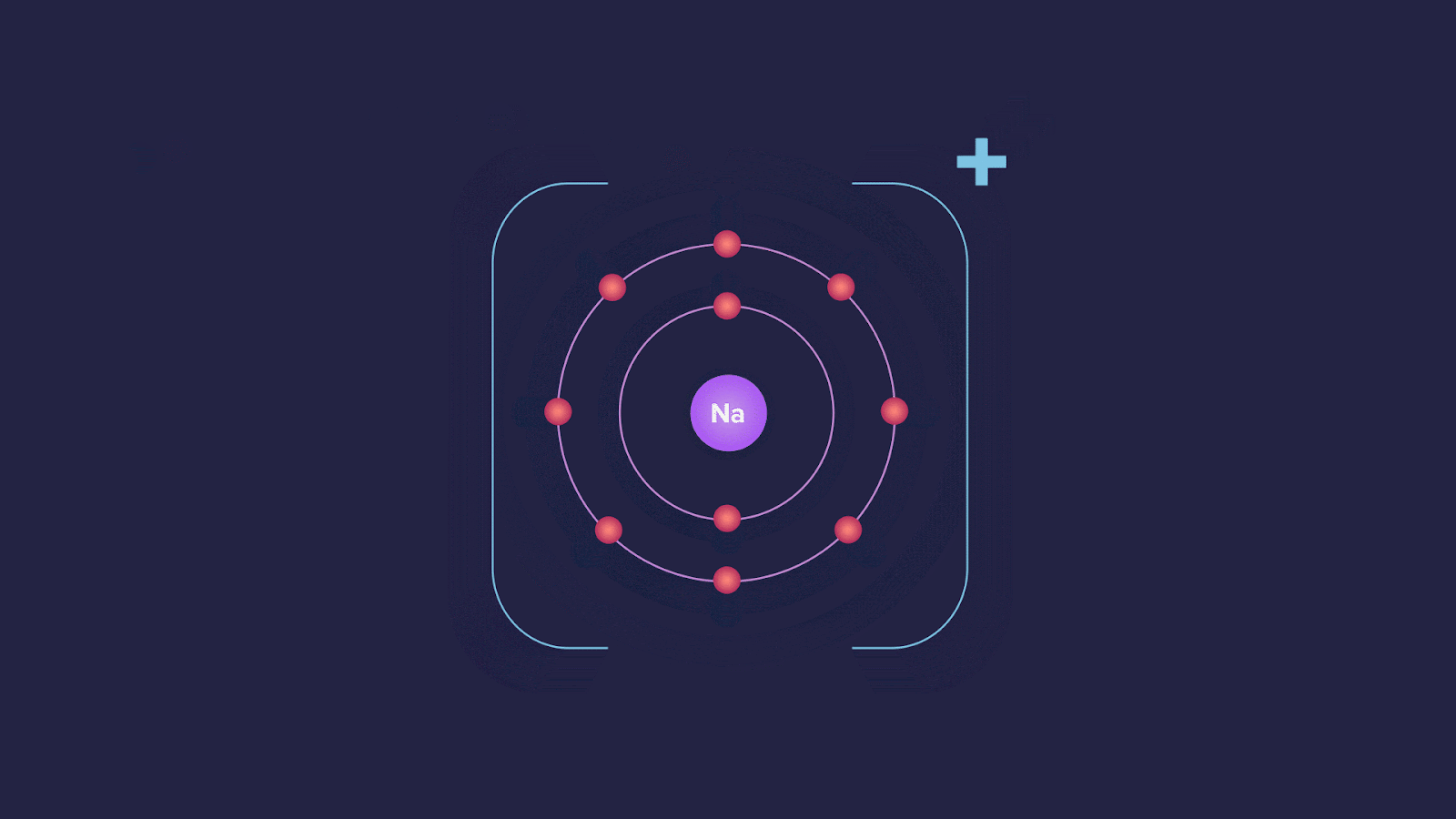
It is the quantitative measure of the tendency of an element to lose an electron. Smaller the ionisation energy, the easier it is for the neutral atom to lose an electron and become a positive ion.
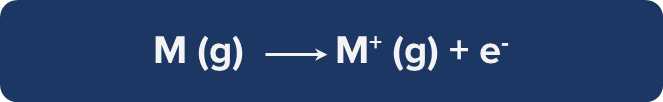

General Trends in Ionisation Energy Across the Periodic Table
Variation of Ionisation Energy Across a Period
- The atomic radius reduces as the number of electrons rises, but the number of shells stays the same when moving from left to right throughout a period.
- Therefore, when atomic size reduces, the nucleus's and the valence electrons' attractive force grows.
- Ionization energy thus typically rises from the left to the right of a period.
Example: As we go from Li to Ne, the first ionisation energy of the second-period elements rises. The size shrinks and the nuclear force of attraction between the nucleus and the valence electron strengthens as we go from Li to F.
Variation of Ionisation Energy Down the Group
- The drop in ionisation energy of elements as they move down a group is brought on by an increase in atomic size.
- The outermost electrons distance themselves from the nucleus as the number of shells rises, lowering the effective nuclear charge.
- Additionally, the shielding effect rises on moving down the group, which results in a decrease in ionisation energy.
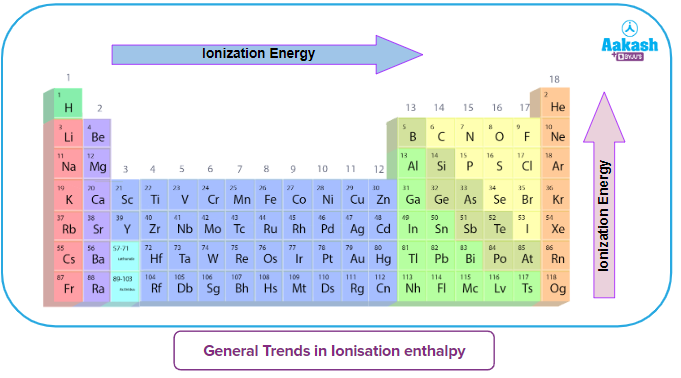
The First Ionisation Energy of the First 60 Elements in the Periodic Table
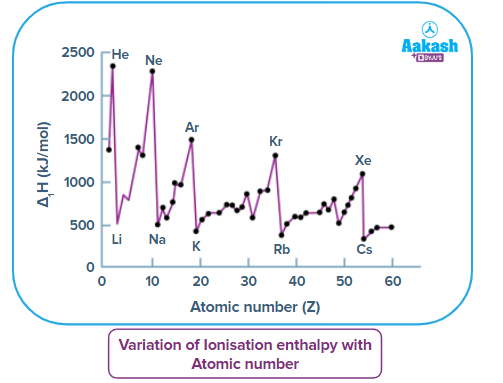
Analyzing the provided graph, we discover
Noble gases (He, Ne, Ar, Kr, Xe)
- Due to their fully filled orbitals, noble gases have the highest ionisation enthalpies in their respective periods. The removal of the outermost electron greatly reduces the stability of noble gases. Noble gases have a high ionisation energy as a result.
- Due to the growth in atom size as one moves down the group (Group 18), the graph shows a drop in the ionisation energy values of noble gases.
Alkali metals (Li, Na, K, Rb, Cs)
- Alkali metals have the lowest ionisation energy in their respective periods. This is because alkali metals would achieve a stable noble gas structure after losing one electron.
- The ionisation energy of alkali metals decreases as one moves down the group (Group 1). Ionisation energy is quite low for caesium (Cs). As a result, solar cells use it.
Successive Ionisation Energies
First Ionisation Energy: It is the amount of energy necessary to expel the final electron from a gaseous neutral atom that is in its ground state.
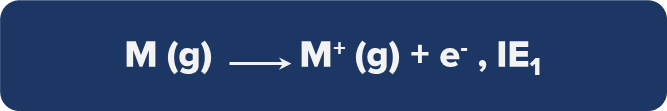
Example:
![]()
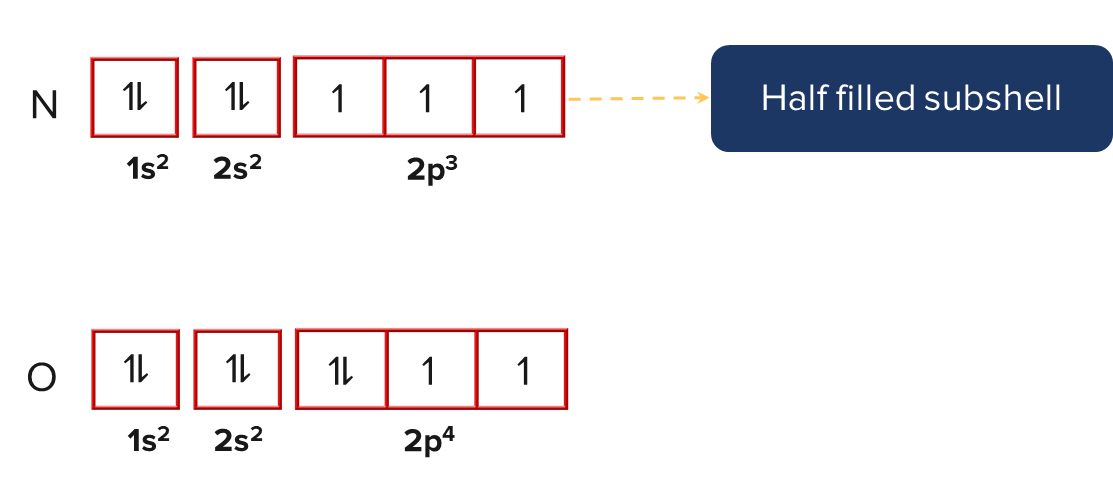
In the aforementioned case, beryllium's first ionisation energy is higher than boron's. This is due to the fact that in the case of beryllium, the outermost electron is located in the s-orbital, which is more penetrated towards the nucleus. Since the p-orbital of boron is less penetrated than the s-orbital, the outermost electron is located there. As a result, it required more energy to remove an electron from an s-orbital than a p-orbital.
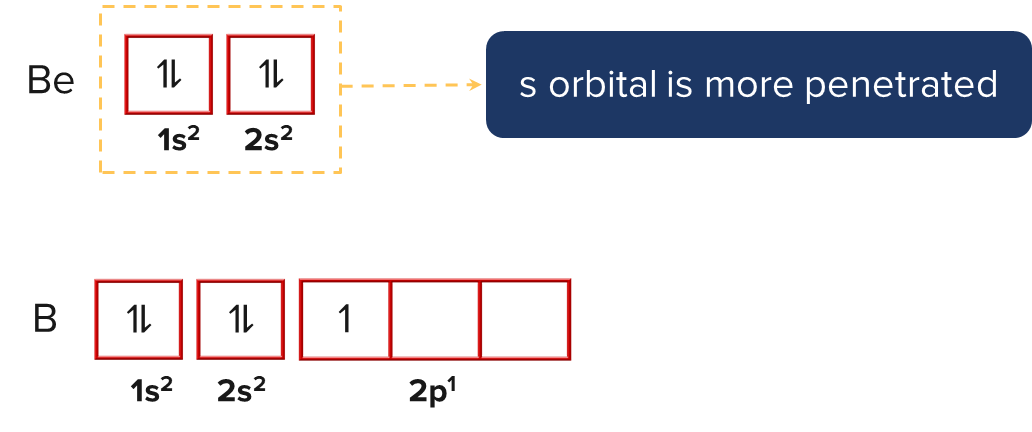
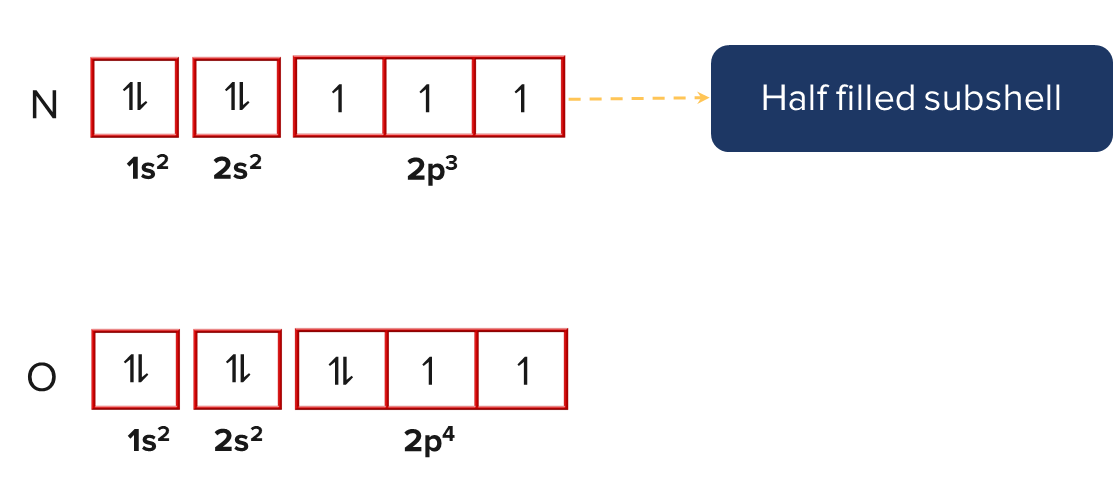
In a similar vein, nitrogen has higher first ionisation energy than oxygen. This is due to the half-filled 2p orbital in nitrogen, which makes it more stable than the oxygen's partially filled 2p orbital.
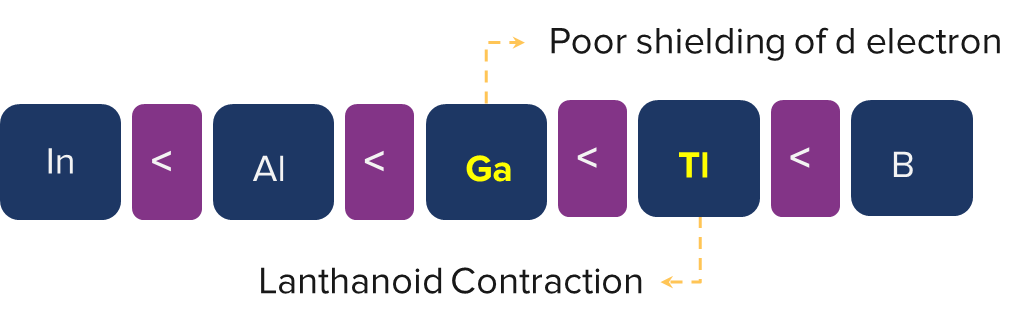
The first ionisation energy of thallium in the 13th group is higher than that of gallium. This is because the inner d-electrons in thallium and the lanthanoid contraction in gallium do a poor job of shielding the outer electrons.
Second Ionisation Energy: It is the amount of energy needed to expel an electron from a mono-positive isolated gaseous ion.
![]()
Third ionisation Energy: Third ionisation energy is the amount of energy needed to free the third most loosely bound electron.
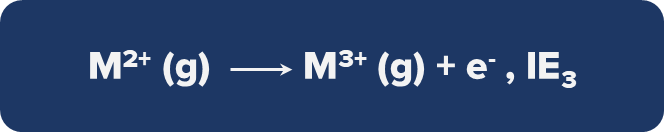
The removal of an electron from a cation is more challenging for an isolated atom/ion than the removal of an electron from a neutral atom.
Therefore,

Generally speaking, non-metals have higher ionisation energies than metals. The maximum ionisation energy value for a particular time period is also found in noble gases. Caesium is utilised in photoelectric cells because it has the lowest ionisation energy.
Factors Affecting Ionisation Energy
- Size of the Atom
- Effective Nuclear Charge (ZEff)
- Screening Effect
- Penetration Effect
- Electronic Configuration
Size of the Atom: Ionization energy falls as atom size increases. This is due to the fact that as the size grows, so does the separation between the outermost electron and the nucleus. The process of removing the electron from the atom so requires less energy.

Effective Nuclear Charge (ZEff): The nucleus and outermost electron are attracted to one another more strongly as the effective nuclear charge rises. As a result, it takes more energy to remove an electron from an atom.

Screening Effect: The ionisation energy decreases as the screening effect in an atom increases. This is due to a decrease in the force of attraction between the nucleus and the outermost electrons as the inner electrons shield the outermost electrons from the nucleus. So, it takes less energy to remove an electron from an atom.

Penetration Effect: Ionization energy rises in direct proportion to the penetration of electrons into various orbitals. The energy needed is in the following order to remove electrons from orbitals in the same shell: s>p>d>f. s- orbital is more penetrated towards the nucleus since it is closer to the nucleus. As a result, removing electrons from p, d, and f orbitals is simpler than from s orbitals.

Electronic Configuration: The stability of partially filled and fully filled degenerate orbitals is quite great, according to Hund's rule. As a result, more energy was needed to remove electrons from orbitals that were either partially or completely filled.
For fully filled, half-filled, and partially filled orbitals, the sequence of IE is as follows.

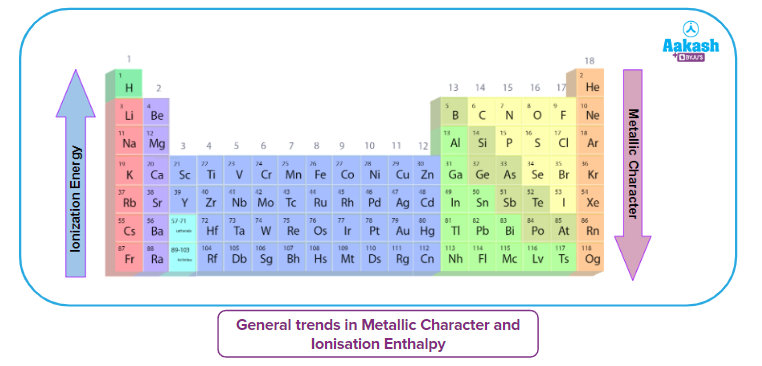
Metallic Character and its Relation with Ionisation Energy
The metallic character describes a metal's degree of reactivity. In chemical processes, metals frequently lose electrons.
- As ionisation energy increases, the metallic character decreases.
- The upward arrow represents an increase in metallic character down the group as ionisation energy lowers (as indicated by the downward arrow).
- Alkali metals have the lowest ionisation enthalpies while noble gases have the highest ionisation enthalpies in the periodic table's long form. Helium (He) has the highest ionisation energy and Caesium (Cs) has the lowest.
- A substance's non-metallic nature reveals its propensity to receive electrons. They often have high electronegativity.
Applications of Ionisation Energy
The ionisation energy can be used to describe patterns in an element's metallic and non-metallic nature.
For instance, non-metals have a high ionisation energy and prefer to create anions, whereas metals have a low ionisation energy and are more ready to produce cations.
- The number of valence electrons in an atom can be determined using the information on ionisation energy.
- Li, for instance, has I.E.1 and I.E.2 values of 5.4 eV and 75.6 eV, respectively. This shows that the first electron can be taken out more easily than the others. As a result, a lithium atom's valence shell has just one electron.
- Reducing power of an element: Reducing nature increases with the decrease in ionisation energy of an element. The lower the value of ionisation energy, the easier it is to remove an electron from an element. So, it can be used as a reducing agent.
- Basic strength of an element: Lesser the ionisation energy, the easier it will be to remove electrons from an element. Therefore it can donate the electron easily and therefore acts as a strong base. Thus, as ionisation energy decreases, basic strength increases.
Recommended Videos
Introduction to Periodic Table - Classification of Elements & Periodic Properties Class 11 Chemistry
Periodic Properties Class 11 Chemistry One-Shot (Full Chapter Revision) | JEE Main 2022 Exam Prep
Periodic Properties Class 11 Chemistry One Shot | NEET 2022 Chemistry Exam Preparation
20 Most Important Questions from Periodic Properties | Periodic Table NEET Questions | NEET 2022
Practice Problems
1. Which of the following ionisation enthalpies will be the greatest for scandium?
a. 1st ionisation energy
b. 2nd ionisation energy
c. 3rd ionisation energy
d. 4th ionisation energy
Answer: D
Solution: The electronic configuration of scandium is [Ar]3d14s2. Hence, it can release up to three electrons in order to attain the stable configuration of the nearest noble gas. So, the fourth ionisation energy would be considerably high as it would have to break the stable noble gas configuration to knock out the fourth electron.
So, option D is the correct answer.
2. Why is the ionisation energy of beryllium greater than that of boron?
Answer: We know that ionisation energy increase on moving from left to right across a period. Therefore, oxygen is expected to have higher ionisation energy than nitrogen, but nitrogen has higher first ionisation energy than oxygen. This is due to the half-filled 2p orbital in nitrogen, which makes it more stable than the oxygen's partially filled 2p orbital.
3. Which element is expected to have the highest first ionisation potential?
a. Sulphur
b. Oxygen
c. Tellurium
d. Selenium
Answer: B
Solution: Down the group, ionisation energy decreases owing to the decrease in effective nuclear charge. Hence, oxygen has the highest ionisation potential among the other members of its group.
So, option B is the correct answer.
4. The ionisation enthalpies of an element are stated as follows:550 kJ mol-1,1560 kJ mol-1, 2680 kJ mol-1, 12590 kJ mol-1. Which group would you expect it to be in?
a. Group 2
b. Group 4
c. Group 3
d. Group 5
Answer: C
Solution: According to the given data, we notice that there is a gradual increase in the ionisation energy values till the third electron. For the fourth electron removal, the ionisation energy increases drastically. Hence, the element should be present in Group 3, having 3 valence electrons.
So, option C is the correct answer.
5. Cations and anions forming ionic compounds should have respectively
a. Higher Ionisation Energy, Lower Electron Affinity
b. Lower Ionisation Energy, Higher Electron Affinity
c. Lower Ionisation Energy, Lower Electron Affinity
d. Higher Ionisation Energy, Higher Electron Affinity
Answer: B
Solution: Metals should readily lose electrons to form cations and hence they should have lower ionisation energy. And anions should readily accept electrons and so should have higher electron affinity. In this way, the two oppositely charged species would form an ionic bond by the electrostatic force of attraction.
So, option B is the correct answer.
Frequently Asked Questions – FAQ
1. What is the general trend of ionisation energy across a period and down the group?
Answer: On going down a group, ionisation energy decreases due to the increase in atomic radii, whereas, across a period, atomic radii decreases and the effective nuclear charge is increased, thus ionisation energy increases from left to right.
2. Are ionisation energy and ionisation enthalpy the same?
Answer: While the ionisation energy is defined at any temperature, the ionisation enthalpy is defined as the energy needed to remove the electron at 0 K. Consequently, they are not always equal in size.
3. Which element has the highest first ionisation energy?
Answer: Helium has the highest first ionisation energy (2370 kJ mol-1) and caesium has the lowest first ionisation energy (376 kJ mol-1). Ionisation energy increases from left to right of a period and decreases down the group.


