-
Call Now
1800-102-2727
Initial Rate Method - Rate of a Reaction, Types, Initial Rate, Application, Practice Problems and FAQs
Any moving object is characterised by its speed, ie the distance covered in a unit of time. In vehicles, we know them from the speedometer present in the vehicle.
You might also will be knowing that chemical reactions also take different time intervals to convert into a product. There are very slow to very fast reactions.
You can keep graphite for years, it will never change to diamond, though the thermodynamics predicts it is spontaneous conversion requiring low energy. Radioactive decay of heavy elements takes place but takes years. You might have a curdy white precipitate of silver chloride instantaneously on mixing aqueous silver nitrate and barium chloride. How to quantify and measure the speed (or correctly RATE) of these reactions.
There is a branch in chemistry that deals with the rate or speed with which the reaction takes place and is named chemical kinetics.
Now, you know that chemical reactions can be slow, medium or fast. What factors decide the speed of the reaction? Come, we shall find answers to these questions.
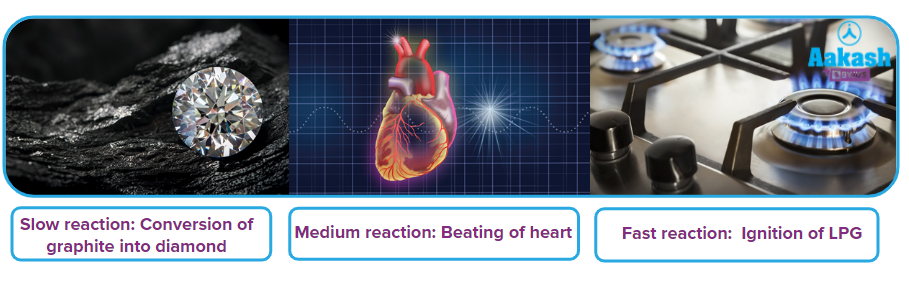
Table of Contents
- Rate of a Reaction
- Types of Rate of Reaction
- Initial Rate of Reaction and Method of Determination
- Application of Initial Rate Method
- Practice Problems
- Frequently Asked Questions-FAQs
Rate of a reaction
The rate of a chemical reaction, is defined as the change in the concentration of reactants or products in unit time, at a given temperature.
For a reaction, where a reactant A is converted into the product B, the reaction is represented as A → B
Rate of a chemical reaction = r = - dAdt = dBdt: where dA and dB are the change in concentrations of A and B in a time interval ‘t’ seconds.
The rate of a reaction is either zero or positive and never negative. Since the concentration of A will be decreasing with the progress of the reaction, a minus sign is included on all reactant concentrations to make the rate positive.
For multi-reactants and multi-products reaction like, aA + bB +....→ cC +dD +...
Rate of the reaction = r =
The rate of the reaction, for a given reactant, changes with the concentration of reactants, pressure, temperature, etc.
Peter Waage and Cato Guldberg, both suggested a theory of the law of mass action, which states that the rate of a chemical reaction at any chosen temperature is proportional to the activity of reacting substances each raised to the power of their respective coefficients. The activity of the substances for practical purposes can be taken as their molar concentration.
For the chemical reaction, aA + bB +....→ cC +dD +...
As, per the Law of mass action, the Rate of a reaction ∝ [A]x[B]y.......;
where [A] and [B] are the molar concentration of reactants A and B.
x and y are some integers called the order of reaction with respect to A and B respectively. They can value zero, fractions and whole numbers. They can be the same or different from the stoichiometric coefficient of A and B and has to be experimentally determined.
Adding a proportionality constant, ‘k’, called the rate constant, to the rate equation,
Rate of a reaction =-[B]y……….(i)
The equation (i) is termed as a differential rate equation.
Types of Rate of Reactions
Rate of the reaction is an experimental quantity. A measurable quantity like concentration, colour, density, optical activity, pressure, and volume that changes with the progress of the reaction is measured with respect to time and plotted on a graph with time on the x-axis and concentration on the y-axis.
As the concentration of the reactants and products are changing with time, the reaction rate also will change with time. As the concentration of reactant will be high at the start, the reaction rate at the start or initial rate of reaction will be very high and will be lowest at the end.
Threes types of reaction rates are used in describing the rate of any chemical reaction:
- Initial rate of reaction
- Instantaneous rate of reaction and
- The average rate of reaction
Initial Rate of Reaction and Method of Determination.
The initial rate of reaction, as the name suggests is the rate of the reaction at the start ie when the concentrations of reactants are maximum and the concentration of product is zero. Changes in variables measured for a very short period of time close to the start of the reaction give the initial reaction rate. But the time period is difficult to fix and calculations based on a single measurement could be erroneous.
Determination of initial reaction rate:
Measurable properties that change proportional to the progress of the reaction is measured experimentally with time and plotted on a graph.
A tangent to the curve at zero point of time is made on the curve. Slope of the curve, gives the initial rate of reaction at the experimental conditions for the given concentrations.
Applications of Initial Rate Method
Initial rate determination is useful to calculate the order and rate constant of the reaction.
- Calculation of partial and total order of the reaction.
In multimolecular reaction like,
aA + bB +....→ cC +dD +...
Rate of a reaction [B]y……….(i)
Where, x, y, etc, are called partial order of the reaction, with respect to the reactants A, B,.......
The sum of the partial order of all the reactants is the total order of the reaction.
Total order of the reaction = x + y +.....
The initial order of the reaction is determined by changing the concentration of one reactant while keeping the concentration of all other reactants constant. Ratios of the initial rates will give the partial order of the changing reactant. Similarly, the partial order of each of the reactants can be determined.
Example: Calculate the partial and total order from the initial rate given for the reactants A and B given below.
A + B → C + D
|
Experiment No. |
[A] |
[B] |
Initial rate (Moles/s) |
|
1 |
0.2 |
0.1 |
1.2x10-3 |
|
2 |
0.2 |
0.2 |
4.8x10-3 |
|
3 |
0.4 |
0.1 |
2.4x10-3 |
|
4 |
0.4 |
0.2 |
9.8x10-3 |
Since, Rate of a reaction [B]y…
Rate of a reaction 1 = r1=k [0.2]x [0.1]y = 1.2x10-3 …….. 1
Rate of a reaction 2 = r2=k [0.2]x [0.2]y = 4.8x10-3 ……....2
Rate of a reaction 3 = r3=k [0.4]x [0.1]y = 2.4x10-3 ……….3
Rate of a reaction 4 = r4=k [0.4]x [0.2]y = 9.6x10-3 ………4
Dividing equation 2 by 1, r2r1
= 2y= 4 or y=2
Dividing equation 3 by 1, r3r1 =
= 2x= 2 or x=1
Partial order of A is 1 and of B is 2.
Rate of a reaction [B]2
Overall order of the reaction = 1 + 2 =3
- Calculation of rate constant:
Substitution of the concentration and order of the reaction in the initial rate equation will give the rate constant of the reaction.
Example: Calculator the rate constant of the reaction discussed above.
Taking the first experimental value and substituting the partial order of the reaction,
Rate of a reaction 1 = r1=k [0.2]1 [0.1]2 = 1.2x10-3
Practice Problems
Q1. For the reaction, 2N2O5 4NO2 + O2the rate can be expressed in two ways:d[N2O5]dt =k[N2O5] and d[NO2]dt =k'[N2O5]
k and k' are related as:
(A) k =k'
(B) k =2k'
(C) 2k =k'
(D) k =4k'
Answer: (C)
For the given reaction, 2N2O5 4NO2 + O2,the rate of reaction can be expressed as follows in terms of both reactants and products:
Rate of a reaction (R)
Given:
Substituting the values of -d[N2O5]dt and d[NO2]dt in the equation (i), we get;
⇒ 2k =k'
Q2. Considering a reaction:
H2 + I2 2HI
This reaction is a second-order reaction.
The differential rate equation for the reaction H2 + I2 2HI can be expressed as:
(A) -d[H2]dt= k [H2] [I2]0
(B) -d[H2]dt= k [H2]0 [I2]0
(C) d[H2]dt= k [H2]2 [HI]0
(D) -d[H2]dt= k [HI]2
Answer: (D)
Solution:
Rate of a reaction can be expressed as
Rate of reaction
∵ The reaction (H2 + I2 2HI) is a second-order reaction.
So, the rate of the reaction would be:
From equations (i) and (ii), we get;
Q3. In the given chemical reaction, CHCl3+ Cl2 CCl4 + HCl, Which of the following statements is correct regarding the rate expression for the reaction?
(A) Rate=k[CHCl3][Cl2]
(B) Rate=k[CHCl3]
(C) Rate =k[Cl2]
(D) Cannot be predicted from given data
Answer: (D)
Solution: As we know that rate law expression can only be determined experimentally.
∴ Rate law expression cannot be predicted from the above-given data for the mentioned reaction.
Q4. For the reaction, A+ B products, it is observed that:
On tripling the initial concentration of A only, the rate of reaction is tripled and on tripling the initial concentration of both A and B, the rate of a reaction is increased by 27 times.
The rate of reaction can be expressed as
(A) Rate = k[A][B]
(B) Rate = k[A]2[B]
(C) Rate = k[A][B]2
(D) Rate = k[A]2[B]2
Answer: (C)
Let’s consider for the reaction, A+ B products, rate is:
Rate(R)=k [A]x [B]y.........(i)
On tripling the concentration of A, the rate also gets tripled.
3R = k[3A]x[B]y.........(ii)
On dividing the equation (ii) by (i), we get;
⇒ 3 = 3x
⇒ x = 1
Also, on tripling the concentration of A and B, rate increases by 27 times.
27R = k[3A]x[3B]y.........(iii)
On dividing the equation (iii) by (i), we get;
⇒ 27 = 3x × 3y
Substituting, x = 1
⇒ 27 = 31× 3y
⇒ y = 2
Substituting the values of x and y in equation (i), we get;
Rate(R)=k [A]x [B]y.........(i)
Rate=k [A]1 [B]2
Frequently Asked Questions-FAQs
Q1. What is meant by the differential rate equation?
Answer: Differential rate laws are used to express the rate of a reaction in terms of the changes in reactant concentrations [d[R]) over a small interval of time (dt)].
It is expressed as:
Differential rate law can also be used to calculate the instantaneous rate of a reaction, which is the rate of reaction defined for an infinitesimally small interval of time.
Q2. What will be the unit of rate constant in case of a gaseous reaction?
Answer: Consider a chemical reaction occurring in the gaseous phase;
xX(g) yY(g)
Rate=k pXn
Where pX is the partial pressure of reactant X and n is the experimentally determined order of this reaction.
⇒ k=RatepXn
Q3. What is the difference between 'Keq' and 'k'?
Answer: 'Keq' is an equilibrium constant, it tells the net extent of reversible reactions whereas k is the rate constant of the reaction that tells how fast a reaction is proceeding.
Q4. Does rate constant change with the rate of a reaction?
Answer: For any reaction, aA + bB cC
Rate of reaction ∝ [A]x [B]y
Rate = k [A]x [B]y
k is rate constant.
x and y are the orders with respect to the reactants A & B respectively.
As the concentration of reactants [A] and [B] changes, the rate of reaction changes but the rate constant (k) always remains constant throughout the entire span of reaction at a given temperature.


