-
Call Now
1800-102-2727
Hydroxylation- Hydroxylation of Alkenes via m-CPBA, KMnO4, OsO4, Hydroxylation of Alkynes, Practice Problems and FAQs
Vicinal Diketones (VDKs) are a group of flavouring substances that can be found in beer. The most prevalent VDKs are 2,3-butanedione (also called diacetyl) and 2,3-pentanedione. While pentanedione gives the beer more honey-like overtones, diethyl gives the beverage sweet butter, caramel, or butterscotch flavours and aromas.
Can someone, however, explain to us how to create them in our chemistry lab? Alkynes can be hydroxylated with oxidizing chemicals like potassium permanganate to create vicinal diketones. Similar to this, alkenes undergo hydroxylation by reacting with various oxidizing agents, producing beneficial byproducts that are utilized in our industries. Let's discuss this in more depth.

Table of Contents
- Hydroxylation of Alkene
- Hydroxylation via m-CPBA in presence of acidified water
- Hydroxylation via potassium permanganate (Baeyer’s Reagent)
- Hydroxylation via osmium tetroxide
- Hydroxylation of alkyne
- Baeyer’s Test
- Practice Problems
- Frequently Asked Questions-FAQs
Hydroxylation of Alkene
During hydroxylation, the alkene's carbon-hydrogen bond oxidizes to a carbon-hydroxyl bond. An oxidation reaction is alkene hydroxylation. An agent that raises the oxidation number is known as an oxidizing agent.
Alkenes can be hydroxylated via three different reactions
1. Hydroxylation via m-CPBA in presence of acidified water
2. Hydroxylation via potassium permanganate
3. Hydroxylation via osmium tetroxide in pyridine and then H2S or NaHSO3
- Hydroxylation via m-CPBA in presence of acidified water
Epoxides that can be produced by employing m-CPBA (meta-chloro peroxy benzoic acid) can be cleaved by aqueous acid. The conjugate acid of the epoxide is created by proton transfer from the acid catalyst and is attacked by nucleophiles like water. The double bond is subsequently anti-hydroxylated as a result. The oxidation status of any of an epoxide's atoms or groups is unaffected by its hydration. The reaction conditions dictate the ring-opening process in epoxides.
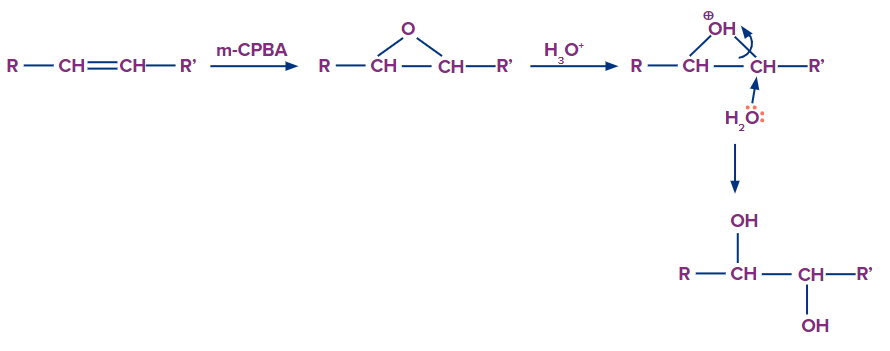
- Hydroxylation via potassium permanganate (Baeyer’s Reagent)
Alkenes can be mildly or strongly oxidized depending on the reaction environment. In neutral permanganate solution, for example, alkenes form vicinal diols.
The generic reactions for the various mild oxidative conditions are summarized below.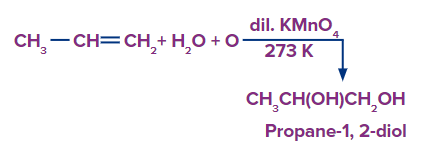
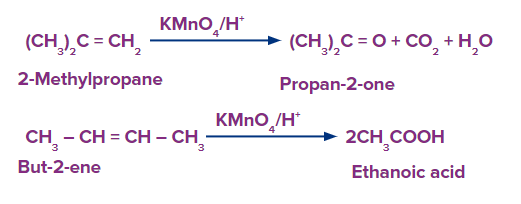
The alkene is cleaved into two products during strong oxidation with acidic potassium permanganate.
If unsaturated carbon is disubstituted, the product is a ketone.
If unsaturated carbon is monosubstituted, the product is a carboxylic acid.
If unsaturation is at the terminal, the product is carbon dioxide and water.
The generic reactions for the various oxidative conditions - strong- are summarized below.
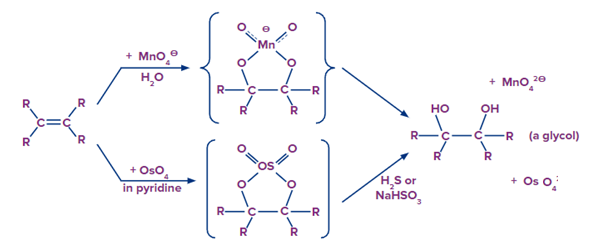
- Hydroxylation via osmium tetroxide
Dihydroxylated products are formed by reacting alkene with aqueous osmium tetroxide in pyridine and then in the second step H2S or NaHSO3 is used to yield the desired product.
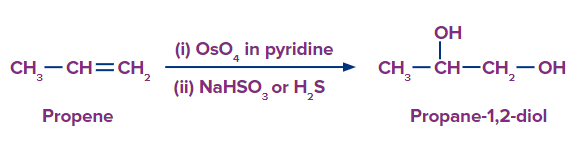
Mechanism
Both reactions appear to be the result of the same mechanism (shown below). We would expect syn-stereoselectivity in the bonding to oxygen based on the mechanism shown here.
Hydroxylation of alkyne
During hydroxylation, the alkene's carbon-hydrogen bond oxidizes to a carbon-hydroxyl bond. An oxidation reaction is alkyne hydroxylation. An agent that raises the oxidation number is known as an oxidizing agent.
In the presence of aqueous potassium permanganate, pyridine reacts to produce dihydroxylated compounds. Based on the mechanism presented here, syn-stereoselectivity in the bonding to oxygen is what we would anticipate.
Baeyer’s Reagent (Potassium Permanganate)
Similar to alkenes, depending on the reaction environment, alkynes can undergo either mild or vigorous oxidation. Alkynes can have more favourable reaction conditions since they are less stable than alkenes. Alkynes can create vicinal dicarbonyls, for instance, in a neutral permanganate solution.
The following is a summary of the general, moderate reactions to the various oxidative situations.

Mechanism:
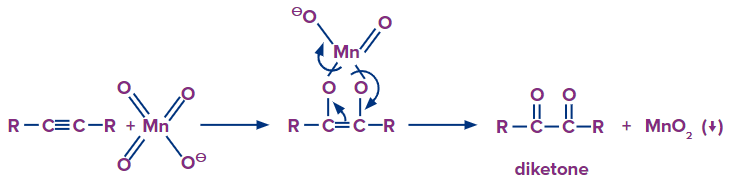
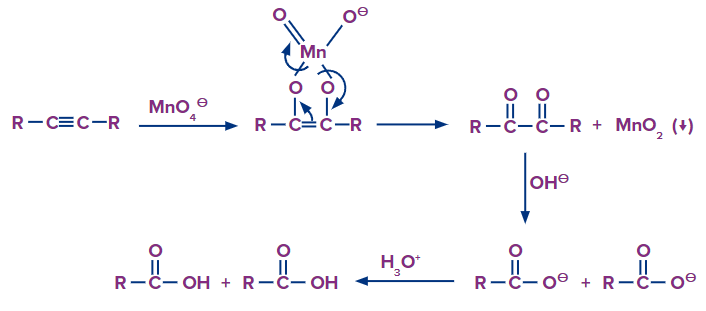
The alkyne is cleaved into two products during strong oxidation with basic potassium permanganate. Because at least one of the reaction products is a carboxylic acid, the acid-base chemistry of the product in the reaction solution must be considered. Carboxylic acids are deprotonated to carboxylates in basic
The generic reactions for the various oxidative conditions - strong- are summarised below.

Mechanism:
Baeyer’s Test
In qualitative organic analysis KMnO4 is used to test for the presence of unsaturation. It is also known as Baeyer's reagent after the German organic chemist Adolf von Baeyer.
The reagent used in Baeyer’s test is an alkaline potassium permanganate solution.
When potassium permanganate is added to an unsaturated hydrocarbon like alkenes and alkynes, the pink colour of potassium permanganate disappears. The colour fades from purplish-pink to brown when it reacts with double or triple bonds (-C=C- or -C≡C-).
Baeyer’s reagent will give brown precipitates with both terminal and non-terminal alkenes and alkynes. Hence, this test can be used for the detection of alkenes and alkynes but does not specify whether they are terminal or non-terminal.
Baeyer’s Reagent Brown precipitates
Baeyer’s Reagent Brown precipitate

![]()
Practice Problems
Q1. Which test can be used to distinguish between cyclohexene and cyclohexane and predict the product of this reaction?
- Ammoniacal cuprous chloride;
- Ammoniacal cuprous chloride;
- Baeyer’s Test;
- Baeyer’s Test;
Answer: (C)
Solutions: Cyclohexene being non-terminal alkyne and cyclohexane being alkane can be distinguished with baeyer’s tests as in option (A) Ammoniacal cuprous chloride give precipitates with terminal alkynes only.
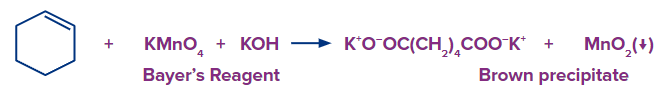
When alkaline potassium permanganate is added to an unsaturated hydrocarbon, the pink colour of potassium permanganate disappears. The colour fades from purplish-pink to brown when it reacts with double or triple bonds (-C=C- or -C≡C-). Baeyer’s reagent will give brown precipitates with both terminal and Non- terminal alkynes. Hence, this test can be used for the detection of alkynes.
Thus, the correct option is (C).
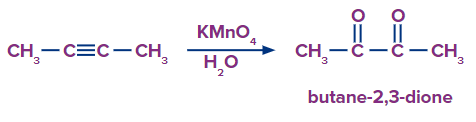
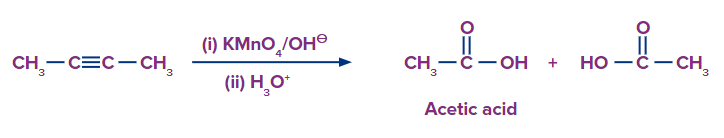
Q2. What would be the expected product when But-2-yne is made to react with
(i) neutral potassium permanganate and acidified water
(ii) alkaline potassium permanganate and acidified water
- Butane-2,3-dione, Butane-2,3-dione
- Butane-2,3-dione, Acetic Acid
- Acetic Acid, Acetic Acid
- Acetic Acid, Butane-2,3-dione
Answer: (B)
Solution: Alkynes, for instance, create vicinal dicarbonyls in a solution of neutral permanganate. Below is a summary of the generic reactions for the various oxidative conditions. So Butane-2,3-dione should be the final result.
Alkyne splits into two products after intense oxidation with basic potassium permanganate. Deprotonation of carboxylic acids produces carboxylates in basic solutions. To protonate the carboxylate to the neutral form of the carboxylic acid, a second reaction step is necessary. Therefore, acetic acid should be the end product. The suggested solution is option (B).
Q3. The centre of a tetrahedral cluster of negatively charged oxygen atoms in permanganate is occupied by the metal element manganese. So, how would such a molecule interact with the pi-electrons of a double bond that are nucleophilic?
Answer: One explanation is that, like platinum, the vacant d-orbital of an electrophilic metal atom stretches far beyond the surrounding oxygen atoms and begins to transfer electrons from the double bond to the metal. The back-bonding of the nucleophilic oxygens to the antibonding *-orbital completes this interaction.
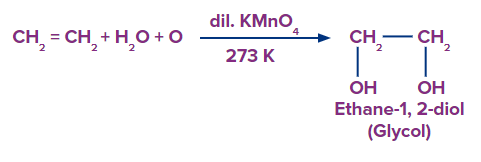
Q4. Pick out the correct statement for the product for the given reaction
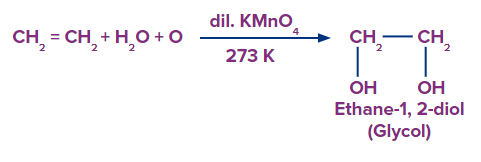
- It is frequently used as antifreeze in the cooling systems of automobiles.
- It is frequently utilised in the production of synthetic fibers.
- The common name of the product is ethylene glycol.
- All of these
Answer: (D)
Solution: Alkenes can be gently or strongly oxidized depending on the reaction environment. In neutral permanganate solution, for example, Alkenes form vicinal diols.
Hence, the product is a vicinal diol, Ethane-1,2-diol. A correct answer is option (D) as all the given statements are correct. The common name of Ethane-1,2-diol is Ethylene Glycol which is used in the manufacturing of synthetic fibres and used as antifreeze for colling systems of automobiles.
Frequently Asked Questions-FAQs
1. Does the process of hydroxylation involve both oxidation and reduction?
Answer: No, the hydroxylation of alkynes is an oxidation process. An agent that raises the oxidation number is known as an oxidizing agent. An oxidizer, potassium permanganate.
2. How may osmium tetraoxide's toxicity be diminished?
Answer: Osmium tetroxide can be prevented by utilizing catalytic amounts of OsO4 and stoichiometric amounts of an oxidizing agent, such as hydrogen peroxide because it is costly and highly hazardous.
3. What is the alkyne hydroxylation reaction?
Answer: A carbon-carbon pi link is changed into two carbon-hydroxyl bonds during the oxidation process known as the "hydroxylation" of alkenes. These bonds are then hydrolyzed to form vicinal diketones. They are also capable of producing carboxylic acids when they are vigorous.
4. What are the uses of alkene?
Answer: They help make plastics like polystyrene, which is used to make refrigerator parts and automobile battery boxes, as well as polythene, which is used to make buckets, bowls, and bags. They are employed in the synthesis of ethane-1,2-diol, which is a component of antifreeze used in car radiators.


