-
Call Now
1800-102-2727
Catalytic Hydrogenation of Alkenes and Alkynes- Hydrogenation of Alkenes and Alkynes, Partial Hydrogenation, Lindlar Catalyst, Practice Problems and FAQs
Could you please tell me, what exactly is the distinction between reduction and hydrogenation?
Chemical reduction occurs when an element adds hydrogen or gains electron(s), causing its oxidation number to decrease. Concurrently, the reducing agent loses electrons, increasing its oxidation number. Hydrogenation, on the other hand, is the addition of specifically hydrogen to a reducible (unsaturated) molecule.
Let us go over catalytic hydrogenation in depth.
Table of Contents
- Hydrogenation reaction of alkenes
- Addition reactions of Alkynes
- Partial hydrogenation (reduction) of alkynes
- Lindlar Catalyst
- Practice Problems
- Frequently Asked Questions – FAQs
Hydrogenation reaction of alkenes
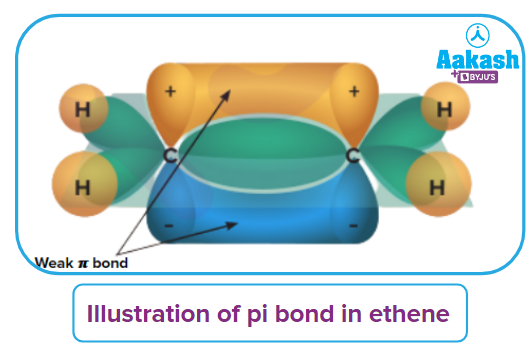
Alkenes are rich sources of loosely held π electrons. Hence, alkenes give addition reactions in which the electrophiles add on to the carbon-carbon double bond to form the addition products. An illustration of the weak π bond in ethene is given. These loosely held π electrons are responsible for the addition reactions in alkenes.
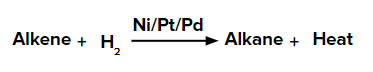
Alkenes adds up one molecule of dihydrogen gas in the presence of finely divided nickel, palladium, or platinum to form alkanes.
This reaction is known as the Sabatier-Sanderson reaction when Ni is used as a catalyst.
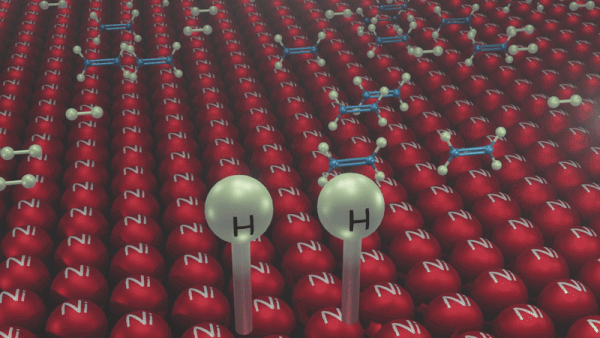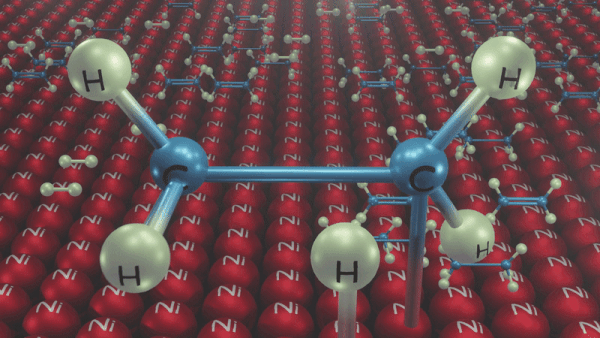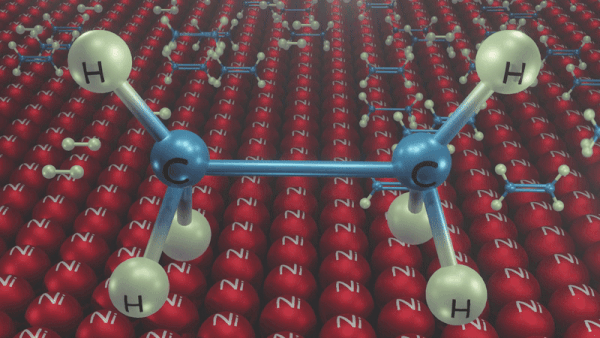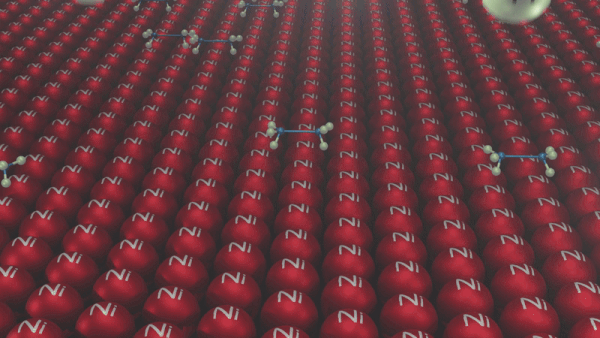
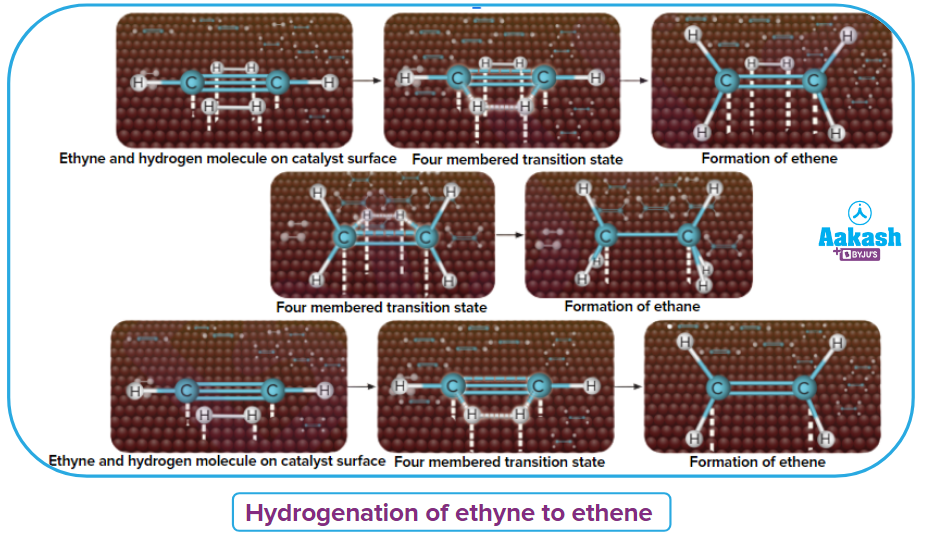
An illustration of the addition of dihydrogen to ethene in the presence of nickel is given, Ethene is adsorbed over the catalyst surface. Syn addition (addition of H2 from the same side) of dihydrogen happens and a four-membered high-energy transition state is formed in the process. Finally, ethene is reduced to ethane.
Addition reactions of Alkynes
Due to the presence of two weak pi (π) bonds, alkynes undergo an addition reaction. Alkynes add up to two dihydrogen molecules, halogens, hydrogen halides, etc.
An illustration of orbital overlap in alkynes representing the weak pi (π) electron cloud is given.
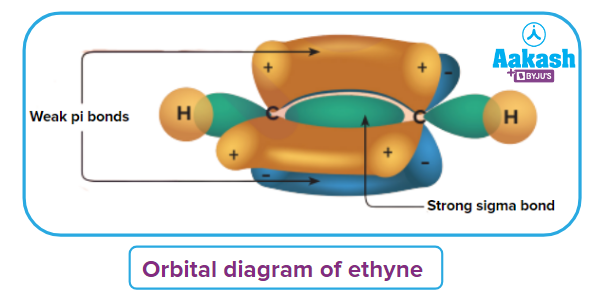
General addition reaction of alkynes
Let us consider the addition of any compound (HZ) to an alkyne.
Step 1: Addition of electrophile
When we add one equivalent of an electrophile (let’s say H+) to an alkyne, it forms a vinyl carbocation. This is an electrophilic addition reaction.
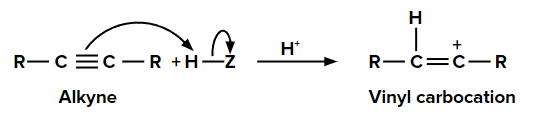
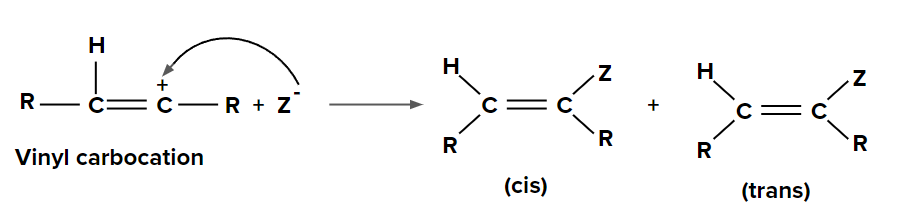
Step 2: Addition of nucleophile (Z-) to vinyl carbocation
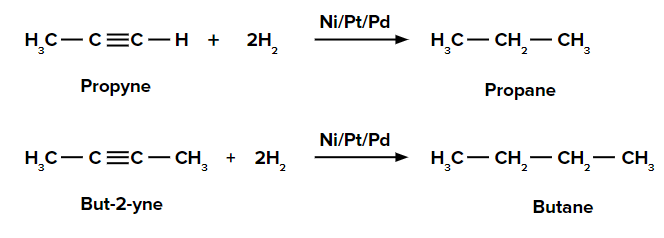
(i) Catalytic Hydrogenation of Alkyne
An addition of dihydrogen to alkynes in the presence of Ni/Pd/Pt gives alkanes.
For example,
Partial hydrogenation (reduction) of alkynes
- While adding H2 to alkynes, if the reduction is to be stopped at the alkene stage, then the
catalyst needs to be poisoned.
- Palladium catalyst (Pd/CaCO3) deliberately poisoned with lead or sulfur compounds or quinoline, etc., is known as Lindlar’s catalyst.
Lindlar Catalyst
Lindlar catalysts are heterogeneous catalysts composed of catalytically active palladium on support of, calcium carbonate, and one of the variety of catalyst poisons (such as quinoline or lead oxide). To produce an alkene from an alkyne, a Lindlar catalyst can be used to catalyse the alkyne's hydrogenation (reaction of the alkyne with molecular hydrogen, H2). This catalyst was created by the British chemist Herbert Lindlar.
Composition
Lindlar catalyst is made up of three main components:
- Active metal palladium (5 per cent of the total weight)
- Calcium Carbonate (over which the palladium is deposited- a support)
- Poisonous catalysts (typically lead salts and quinoline)
Lindlar Catalyst Catalytic Action
The Lindlar catalyst facilitates the addition of hydrogen to the alkyne in palladium-catalyzed hydrogenations of alkynes to yield the corresponding alkene. When this catalyst is used, the hydrogen substituent is always added via syn addition (addition of the substituent on the same side of the bond). As a result, the final product is entirely composed of the alkene's cis-isomer.
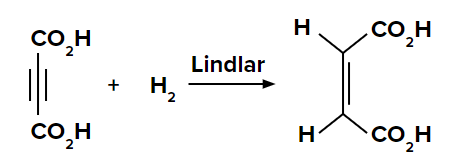
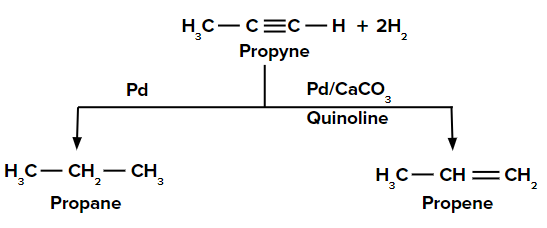
Reduction of propyne to propane and propene
In the presence of a palladium catalyst, propyne is reduced to propane. When the reduction is carried out with palladium poisoned with quinoline, propyne is reduced to propene.
An illustration for the hydrogenation of ethyne is given. On the catalyst surface (Pd), ethyne is converted to ethane when two moles of H2are added. A four-membered cyclic transition state is involved in this process. When the palladium catalyst is deactivated using poisons, then ethyne is hydrogenated to ethene.
Practice Problems
- Which of the following will react fastest with the H2 under catalytic hydrogenation conditions?
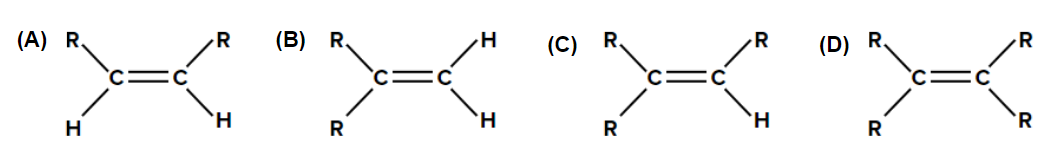
Answer: We know that the rate of hydrogenation is inversely proportional to the steric crowding around
the double bond in alkenes.
- Syn addition of H2 will be the fastest in alkene (A) as it has the least steric crowding.
- In (A), dihydrogen can be approached from the side of the alkene, where two hydrogen atoms are attached (i.e., less steric hindrance). Hence, it will undergo the fastest catalytic hydrogenation.
Hence, option (A) is the correct answer.
2. In the presence of a platinum catalyst, hydrocarbon A adds hydrogen to form n-hexane. When hydrogen bromide is added to A instead of hydrogen, only a single Bromo compound is formed. Which of the following is A?
- CH3-CH2-CH=CH-CH2-CH3
- CH3-CH2-CH2-CH=CH-CH3
- Both (a) and (b)
- CH2=CH-CH2-CH2-CH2-CH3
Answer: As CH3-CH2-CH=CH-CH2-CH3 (hex-3-ene) is a symmetrical alkene, only one product will be obtained on the addition of HBr, i.e., 3-Bromohexane.
As CH3-CH2-CH2-CH=CH-CH3 (hex-2-ene) is an unsymmetrical alkene, more than one product is formed. Thus, 2-Bromohexane and 3-Bromo hexane will be formed as the products.
• As CH2=CH-CH2-CH2-CH2-CH3 (hex-1-ene) is an unsymmetrical alkene, 2-Bromohexane will be formed as the major product and 1-Bromohexane will be formed as the minor product.
Since only one bromo compound is formed as the product in the case of hex-3-ene. Hence option (A) is the correct answer.
3. When 2-butyne is treated with H2/Pd-CaCO3, what is the product formed?
- cis-But-2-ene
- trans-But-2-ene
- But-1-ene
- Butan-2-ol
Answer: When the hydrogenation of 2-butyne takes place in the presence of H2/Pd-CaCO3, it gives only a cis-alkene. Thus, 2-butyne gives cis-but-2-ene.
Thus, option (A) is the correct answer.
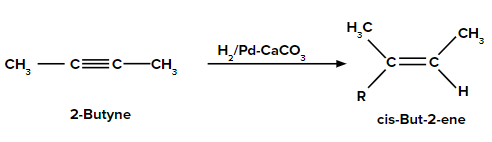
4. Which of the following is product B?
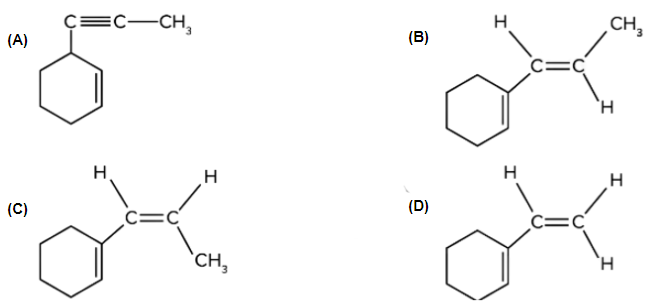
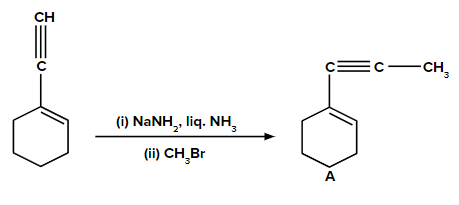
Answer: The given reactant (terminal alkyne substrate) on reaction with sodamide, NaNH2, is deprotonated and gives the conjugate base. The reaction of the conjugate base (acts as a nucleophile) with methyl bromide, CH3Br, produces higher alkyne. The product A is shown below:
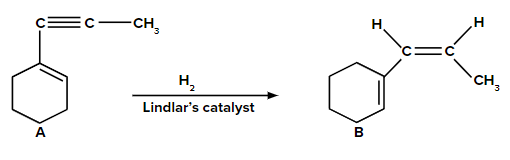
Now, A on reaction with Lindlar’s catalyst converts triple bond (−C≡C−) into cis double bond
(>C=C<). Syn-addition would happen in this conversion. So, product B is as follows:
Therefore, option (C) is the correct answer.
Frequently Asked Questions – FAQs
- What are the applications of Lindlar Catalysts?
Answer: Lindlar catalysts are commercially used in the synthesis of vitamin A. They are also involved in the production of dihydro vitamin K1. With the help of this catalyst, phenylacetylene can be converted to styrene.
- Are Lindlar catalysts homogeneous catalysts?
Answer: Lindlar catalysts are heterogeneous catalysts, which means that the physical phase of the catalyst differs from the physical phase of the reacting entities.
- Why are the lead salts and quinoline in Lindlar catalysts 'Poisoned'?
Answer: Palladium catalysts often have significant catalytic activity and can even reduce double bonds. Using such catalysts, alkanes can be produced during the hydrogenation of alkynes (the alkene products undergo further hydrogenation under the influence of the catalyst). The Lindlar catalyst has been poisoned and is unable to reduce double bonds. As a result, when this catalyst is used in the hydrogenation of alkynes, no alkanes are formed.
- What's the difference between dehydration and dehydrogenation, exactly?
Answer: We've all been puzzled by these terms. The major distinction between hydration and hydrogenation is that hydration includes the addition of water molecules to an organic compound, whereas hydrogenation involves the addition of a hydrogen molecule.


