-
Call Now
1800-102-2727
Oxidative Hydroboration of Alkynes- Hydroboration, Oxidation Reaction of Alkyne, Mechanism, Hydroboration Step, Oxidation step, Practice Problems and Frequently Asked Questions
Are you curious about the ingredients of your favourite perfumes? We'll break down all of those complex components and compounds so you can figure out which scents you really prefer. Today's topic is aldehydes and ketone carbonyl compounds. They appear to be a byproduct of a chemical process, but they're actually an organic component found in a range of natural components, but modern perfumers use synthetic substitutes.
Perfume professionals regularly use aldehydes in their formulations. To put it another way, these components make rose fragrances lighter and more effervescent.
So, in the perfume industry, the preparation of these compounds must play a vital part, and today we will look at one of the methods, which is the hydroboration - oxidation of alkynes.

Table of Contents
- Hydroboration Oxidation reaction
- Mechanism
- Hydroboration Process
- The Oxidation Process
- Practice Problems
- Frequently Asked Questions-FAQs
Hydroboration Oxidation reaction
The hydroboration oxidation reaction is an organic chemical reaction that is used to convert alkenes into primary alcohols or alkynes into ketones or aldehydes. This is accomplished through a two-step procedure that includes a hydroboration step and an oxidation step. This is accomplished through a net addition of water (across the entire double bond).
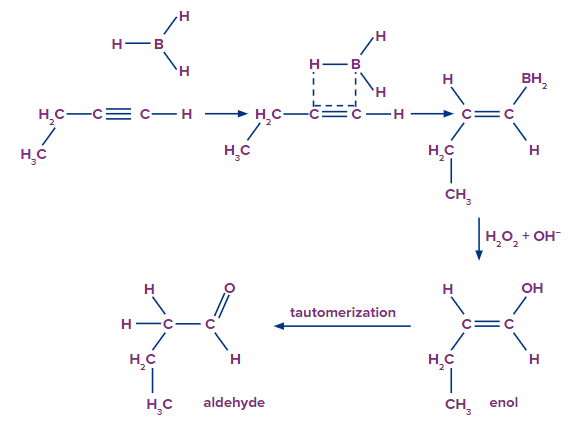
Mechanism
The mechanism of hydroboration oxidation can be thought of as an anti-Markovnikov reaction in which a hydroxyl group attaches itself to the less substituted carbon.
The conversion of alkynes into ketone or aldehyde takes place here. The entire reaction can be broken down into two steps, as explained below.
Step-1: Hydroboration Process
In an anti-Markovnikov way, the alkynes can undergo hydroboration. The less substituted carbon, which is also the least hindered, becomes a priority target for the boron atom's attack. A bulky reagent of borane must be used to stop the reaction at the alkenyl group attached to the borane stage. If borane is used alone, it will result in the hydroboration of both the alkyne's pi bonds.
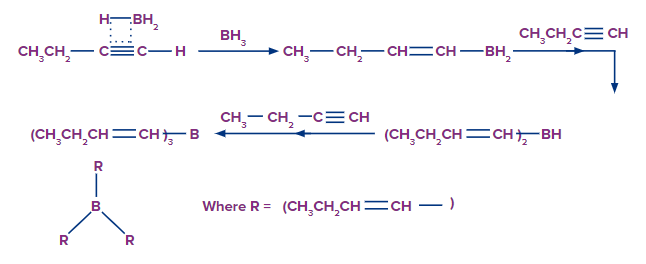
Step-2: The Oxidation Process
Now that the trialkyl borane has been produced, the second step in the hydroboration process can begin. The boron atom is attacked in this step by the hydroperoxide ion, which is nucleophilic in nature. The R group, along with its electron bond pair to the adjacent oxygen atom, is now rearranged.
The ion of hydroxide has now been removed. This process is repeated three times to produce trialkyl borate as the product. This trialkyl borate is now treated with water to produce the required neutral alcohol. This step of the mechanism is illustrated below.
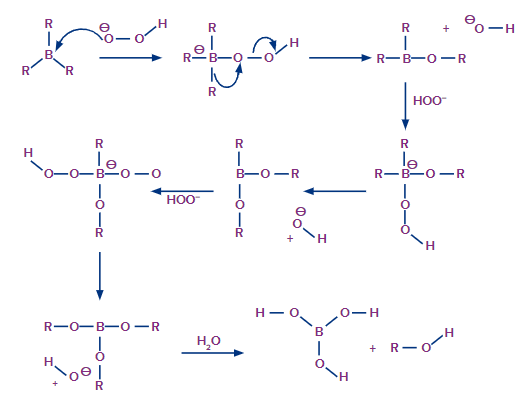
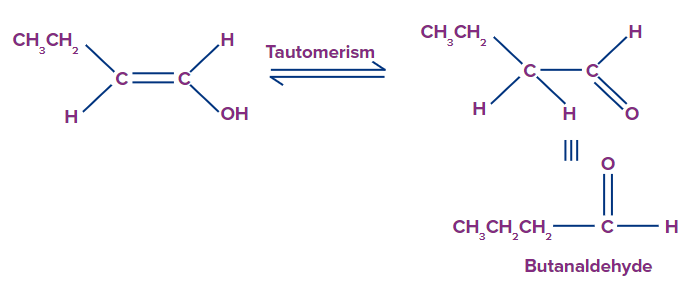
Practice Problems
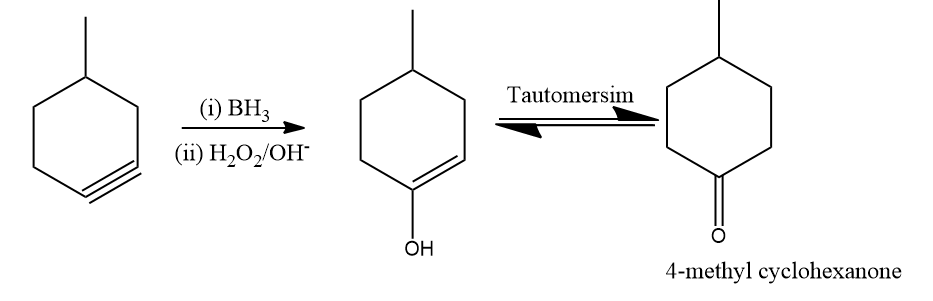
Q1. The expected result of a hydroboration-oxidation reaction involving 4-methyl cyclohexyne would be
A. 4-Methyl Cyclohexan-3-one
B. 4-Methyl Cyclohexan-1-one
C. 2-Methyl Cyclohexan-2-one
D. 4-Methyl Cyclohexan-3-one
Solution: Alkynes are transformed into aldehydes and ketones by the hydroboration oxidation reaction through an organic chemical process. This is made using a two-stage process that includes a hydroboration step and an oxidation step. An anti-Markovnikov Rule is used to achieve a net addition of water.
When methyl cyclohexyne is reacted with BH3 followed by , the expected product according to the Anti Markovnikov rule is 4-Methyl cyclohexan-1-one. Hence, the correct answer is an option (B).
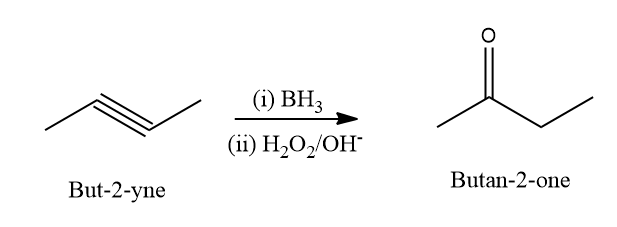
Q2. When But-2-yne undergoes a hydroboration-oxidation reaction, the expected product would be
A. Butan-2-one
B. Butan-3-one
C. Octan-4-one
D. Butanal
Solution: Alkynes undergo an organic chemical reaction known as hydroboration oxidation that results in aldehydes and ketones. This is produced via a two-stage process that includes the steps of hydroboration and oxidation. Net addition of water is accomplished using the anti-Markovnikov Rule.
When But-2-yne is reacted with BH3 followed by , the expected product according to the Anti Markovnikov rule is Butan-2-one. Hence, the correct answer is an option (A).
Q3. In which case will hydroboration oxidation and acid hydration produce different products?
(A) 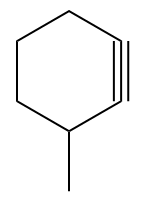
(B) 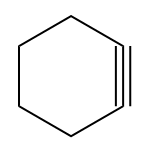
(C) CHCH
(D)
Solution: The hydroboration oxidation reaction converts alkynes to aldehydes and ketones by an organic chemical reaction. A two-stage technique, which involves a hydroboration step and an oxidation step, is used to produce this. Using an anti-Markovnikov Rule, a net addition of water is achieved.
Acid-catalyzed hydration is a chemical reaction in which water is added to an unsaturated substrate while an acid catalyst is present. The hydration of ethene is one example.
Cyclohexyne (option B), (option C) and (option D) all are symmetrical alkyne. Hence, the Markovnikov Rule is not applicable in this case. As products formed from Markovnikov or anti-Markovnikov are the same compound.
In option (A), the unsymmetrical alkyne will yield a different product concerning the reagent and can be shown as
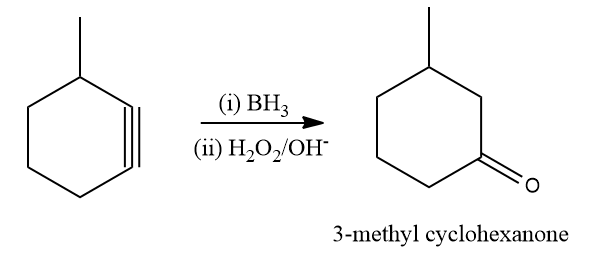
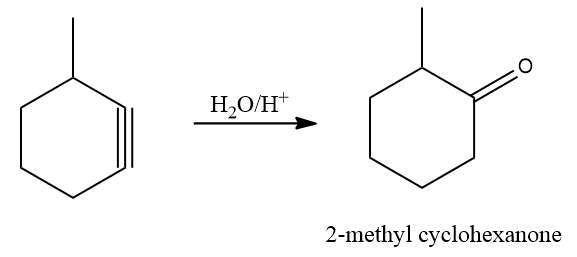
Hence, the correct answer is an option (A).
4. 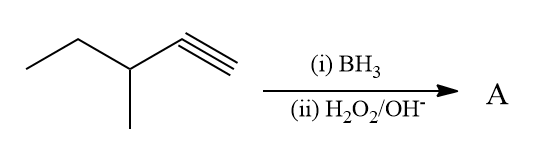
The nature of Product A is
A. Optically Active Aldehyde
B. Optically Active Ketone
C. Optically Inactive Aldehyde
D. Optically Inactive Ketone
Solution: The hydroboration oxidation reaction, which employs an anti-Markovnikov Rule, converts alkynes to aldehydes and ketones via an organic chemical reaction. This is made using a two-stage technique that includes a hydroboration step and an oxidation step.
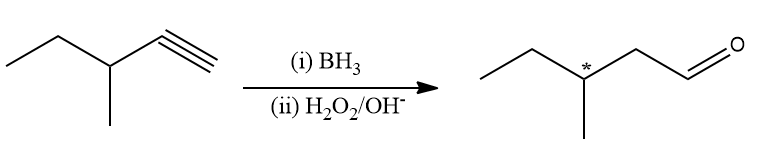
Because third carbon contains one hydrogen, one ethyl, one methyl group and one aldehydic group. The aldehyde produced by this reaction is optically active because carbon-containing four different groups is achiral, a compound is optically active if it has a chiral carbon.
Hence, product A is the primary optically active aldehyde. Hence, the correct answer is (A).
Frequently Asked Questions-FAQs
Q1. What exactly do you mean by "hydroboration"?
Answer: The process of adding the hydrogen boron bond to a double bond between carbon and carbon or carbon and nitrogen is known as hydroboration. It is also possible to perform it on a carbon-carbon triple bond. Some organic compounds can be synthesised using hydroboration.
Q2. Is hydroboration syn or anti-additive?
Answer: The hydroboration mechanism combines hydrogenation and electrophilic addition, and it is a stereospecific (syn addition), which means that the hydroboration occurs on the same face of the double bond, resulting in cis stereochemistry.
Q3. What makes hydroboration so anti-Markovnikov?
Answer: Because the left carbon has an alkyl group and the right carbon has two hydrogens, the hydrogen adds to the side with LESS hydrogens, because boron is bulkier than less hindered carbon, which holds more hydrogen, hydroboration is considered anti-Markovnikov. The action of peroxide on boron-containing carbon. As a result, towards the end, the hydroxide will be added to the LESS substituted carbon, resulting in anti-Markovnikov addition.
Q4. What is the purpose of the alkynes hydroboration product?
Answer: Alkynes hydroboration product ketones and Aldehydes are most commonly utilised as solvents, especially in the explosives, lacquers, paints, and textiles industries. Ketones are also used in tanning, as preservatives, and in hydraulic fluids. Acetone, a liquid with a sweetish odour, is the most significant ketone.


