-
Call Now
1800-102-2727
Classification of Heterocyclic compounds - Aliphatic, Aromatic and Condensed Heterocyclic compounds, Practice problems and FAQs
Have you ever considered how many heterocyclic compounds we encounter on a daily basis?
The majority of the nutrients required for optimum health can be obtained through a balanced diet that includes lots of fruits, vegetables, whole grains, and lean protein sources that are vitamin-rich.
Right?
However, you might be surprised to learn that vitamins, which are crucial micronutrients, are heterocyclic compounds as well.

The majority of medications, pesticides, dyes, and polymers are made of heterocyclic compounds.
So let's get into more depth about them!
Table of Contents:
- Heterocyclic Compounds
- Categorisation of Heterocyclic Compound
- Heterocyclic Aliphatic Compound
- Heterocyclic Aromatic Compound
- Heterocyclic Three-Membered Compounds
- Heterocyclic Four-Membered Compounds
- Heterocyclic Five-Membered Compounds
- Heterocyclic Six-Membered Compounds
- Heterocyclic Condensed or Fused Compound
- Practice Problems
- Frequently Asked Questions
Heterocyclic Compounds:
Heterocyclic compounds are cyclic compounds with a carbon ring and an additional element, such as oxygen, nitrogen, phosphorus or sulphur. The ring of a heterocyclic compound contains at least two distinct elements.
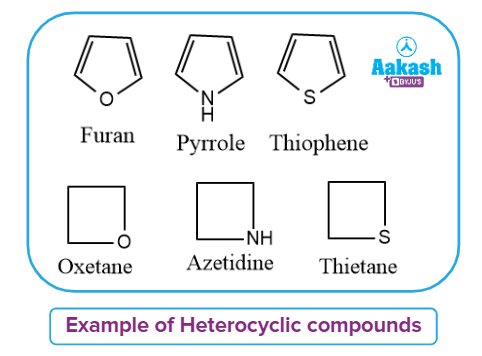
Categorisation of Heterocyclic Compounds:
We can classify Heterocyclic compounds into two categories based on their electronic arrangement:
1. Heterocyclic Aliphatic Compound
2. Heterocyclic Aromatic Compound
Heterocyclic Aliphatic Compound:
The cyclic heterocycles with no double bond are known as aliphatic heterocyclic compounds. Ring strain has a significant impact on the characteristics of aliphatic heterocyclic compounds.
Aziridine, Ethylene Oxide, Thiirane, Oxetane, Azetidine, Thietane, Tetrahydrofuran (THF), Pyrrolidine, Tetrahydrothiophene, Dioxane, Piperidine, and other aliphatic heterocyclic compounds are some examples of heterocyclic aliphatic compounds.
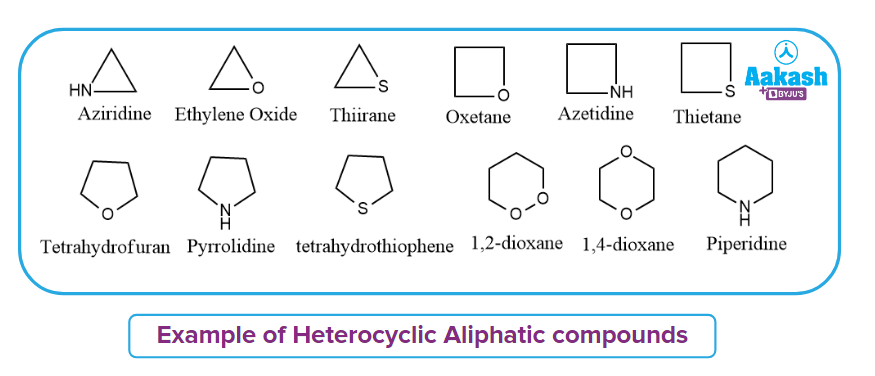
Heterocyclic Aromatic Compound:
Aromatic heterocyclic compounds are cyclic aromatic compounds, as the name implies. Huckels Rule dictates that in order to have aromatic nature, the compound should follow the given guidelines:
- It should have a planar shape.
- There should be no sp3 hybridized atoms in the system.
- It must have a total no. of (4n+2) electrons.
- If one lone pair of sp3 hybridised atom will participate in the conjugation, then we can withdraw the second condition.
Furan, Pyrrole, Thiophene, Oxazole, Pyrazole, Imidazole, Pyridine, Pyrimidine, Pyridazine, Indole, Purine, Quinoline, Isoquinoline, Carbazole and others are examples of heterocyclic aromatic compounds.

Heterocyclic compounds are classified into five types based on their structure:
- Heterocyclic Three-Membered Compounds
- Heterocyclic Four-Membered Compounds
- Heterocyclic Five-Membered Compounds
- Heterocyclic Six-Membered Compounds
- Heterocyclic Condensed or Fused Compounds
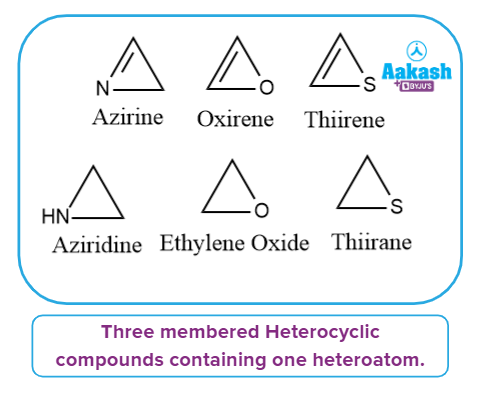
Heterocyclic Three-Membered Compounds:
These heterocyclic compounds have three atoms that can be either saturated or unsaturated.
We can further divide it into two categories based on the number of heteroatoms present:
Heterocyclic compounds containing one heteroatom:
It has one heteroatom in its ring, as the name implies. Azirine, Oxirene, Thiirene, Aziridine, Oxirane, and Thiirane, are some examples.
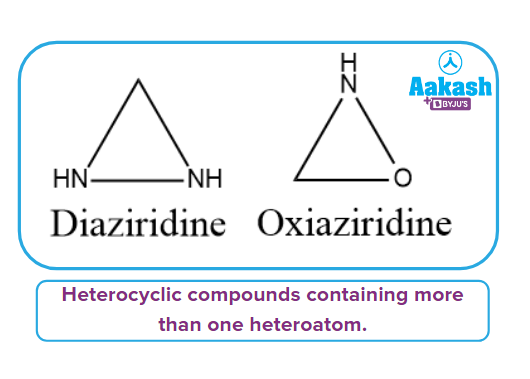
Heterocyclic compounds containing more than one heteroatom:
It has more than one heteroatom in its ring, as the name implies. The ring's heteroatom atoms can be the same or different. Diaziridine and Oxiaziridine are two examples of such types of compounds.
Heterocyclic Four-Membered Compounds:
These heterocyclic compounds have four atoms that can be either saturated or unsaturated.
We can further divide it into two categories based on the number of heteroatoms present:
Heterocyclic compounds containing one heteroatom:
It has one heteroatom in its ring, as the name implies. Azetidine, Oxetane, Thietane, Azete, Oxete, Thiete, and others are examples of such types of compounds.
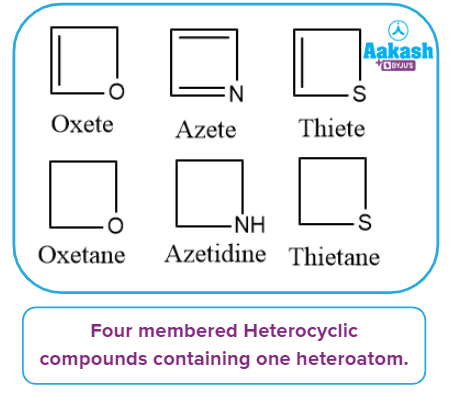
Heterocyclic compounds containing more than one heteroatom:
It has more than one heteroatom in its ring, as the name implies. The ring's heteroatom atoms can be the same or different. Diazetidine and Dioxetane are two examples.
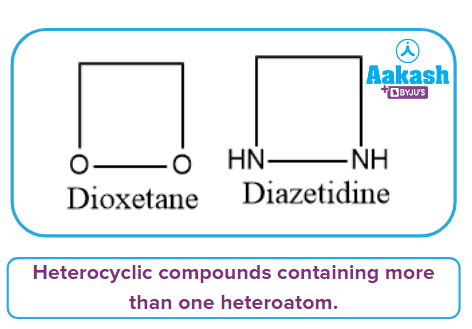
Heterocyclic Five-Membered Compounds:
These heterocyclic compounds containing five atoms can be further divided into two categories based on the number of heteroatoms present.
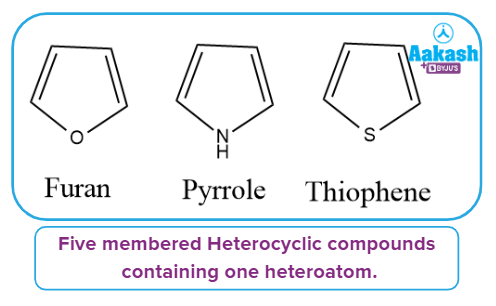
Heterocyclic compounds containing one heteroatom:
It has one heteroatom in its ring, as the name implies. Furan, pyrrole, and thiophene are a few examples of such types of compounds.
Heterocyclic compounds containing more than one heteroatom:
It has more than one heteroatom in its ring, as the name implies. The ring's heteroatom atoms can be the same or different. Pyrazole, Imidazole, Oxazole, Thiazole, Triazole, and Tetrazole are a few examples.

Heterocyclic Six-Membered Compounds:
These heterocyclic compounds are created by replacing one of the carbon atoms in a homocyclic aromatic compounds with a hetero atom having single pair of electrons.
We can further divide it into two categories based on the number of heteroatoms present.
Heterocyclic compounds containing one heteroatom:
It has one heteroatom in its ring, as the name implies. Examples include pyridine, pyran, and thiopyran.

Heterocyclic compounds containing more than one heteroatom:
It has more than one heteroatom in its ring, as the name implies. The ring's heteroatom atoms can be the same or different. Pyridazine, Pyrimidine, Pyrazine, and other examples
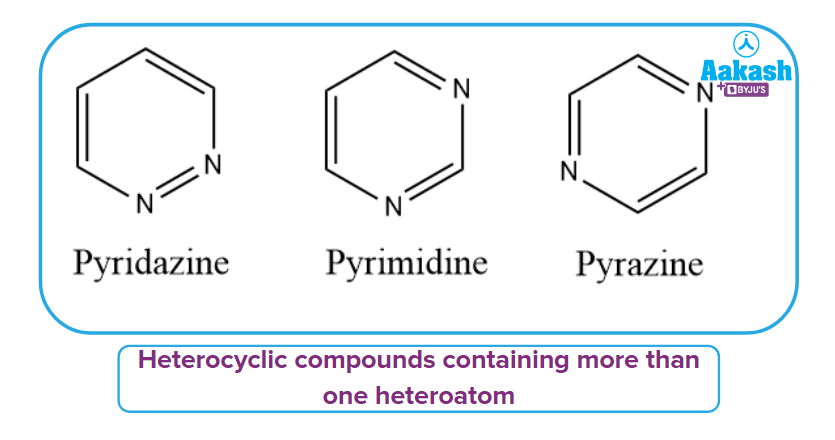
Heterocyclic Condensed or Fused Compound:
Two or more fused rings are present in a condensed or fused heterocyclic compound. Condensed or fused heterocyclic compounds can be carbocyclic or heterocyclic in nature. Indole, Quinoine, Isoquinoline, Carbazole, and others are examples of heterocyclic condensed or fused compounds. Heterocyclic compounds that have been condensed or fused can also be completely heterocyclic. Purine, pteridine and other examples.
Practice Problems:
Q1. Which of the following compounds is not a heterocyclic aromatic compound?
Answer; (B)
Solution: Aromatic heterocyclic compounds are cyclic aromatic compounds, as the name implies. Huckels Rule dictates that it should have a planar shape and must have a total of (4n+2) electrons. There should be no sp3 hybridized atoms in it. If one lone pair of sp3 hybridized atom will participate in the conjugation , then we can withdraw this condition.
In option (A), It has planar structure containing no sp3 hybridized atoms. It contains two different atoms C and N. Lone pairs of nitrogens will not participate in the conjugation , already having 6e-. Hence, All the above conditions are satisfied by the compound. It is a heterocyclic aromatic compound.
In option (B), It has non- planar structure containing sp3 hybridized atom C which will not participate in the conjugation. Hence, It is not a heterocyclic aromatic compound.
In option (C), It has planar structure and contains three different atoms C, O and N. Nitrogens will not participate in the conjugation where as one lone pair of sp3 hybridized atom O will participate in the conjugation , hence having 6e-. Hence, It is a heterocyclic aromatic compound.
In option (D), It has planar structure and contains two different atoms C and N. One lone pair of sp3 hybridized atom N will participate in the conjugation , hence having 6e-. Hence, It is a heterocyclic aromatic compound.
Hence, the correct answer is option (B).
Q2. Which of the following compounds is heterocyclic aromatic compound?
Answer: (C)
Solution: A compound should follow the given conditions in order to become aromatic as per Huckel’s Rule
- It should have a planar shape.
- There should be no sp3 hybridized atoms in it.
- It must have a total of (4n+2) electrons.
- If one lone pair of sp3 hybridized atom will participate in the conjugation , then we can withdraw the second condition.
In option (A), It is not a heterocyclic compound as it contains only carbon atoms in the ring.
In option (B), It has non- planar structure containing sp3 hybridized atoms which will not participate in the conjugation. Hence, It is not a heterocyclic aromatic compound.
In option (C), It has planar structure, no sp3 hybridized atoms in it and contain two different atoms C and N. Lone pair of nitrogens will not participate in the conjugation, as already having 6e-. Hence, It is a heterocyclic aromatic compound.
In option (D), It has planar structure and contains two different atoms C and S. One lone pair of sp3 hybridized atom S will participate in the conjugation , hence having 4e-. Hence, It is not a heterocyclic aromatic compound.
Hence, the correct answer is option (C).
Q3. Which of the following compounds is heterocyclic aromatic compound?
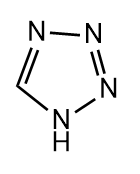
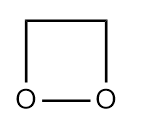
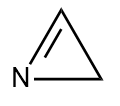
- All of these
Answer: (A)
Solution: Given points should be followed by compound to become an aromatic compound
- There should be no sp3 hybridized atoms in it. If one lone pair of sp3 hybridized atoms will participate in the conjugation, then we can withdraw this condition.
- It should have a planar shape and must have a total of (4n+2) electrons.
In option (A), It has a planar structure and contains two different atoms C and N. One lone pair of sp3 hybridized atom N will participate in the conjugation, hence having 6e- while the other three nitrogen
(sp2 hybridized) will not participate. Hence, it is a heterocyclic aromatic compound.
In option (B), It has a non-planar structure and contains two different atoms C and O. Hence, It is not a heterocyclic aromatic compound.
In option (C), It has a non-planar structure and contains two different atoms C and N but will not participate in the conjugation because of containing sp3 hybridized atom C. In that case, Hence, It is not a heterocyclic aromatic compound.
Hence, the correct answer is option (A).
Q4. Which of the following compounds is a heterocyclic aromatic compound?
Answer: (A)
Solution: Aromatic heterocyclic compounds are cyclic aromatic compounds and should have a planar shape, no sp3 hybridized atoms and must have a total of (4n+2) electrons. If one lone pair of sp3 hybridized atom will participate in the conjugation , then we can withdraw the second condition (no sp3 hybridized atoms ).
In option (A), It has planar structure, no sp3 hybridized atoms in it and contains two different atoms C and N. Nitrogens will not participate in the conjugation, hence having 10e-. Hence, It is a heterocyclic aromatic compound.
In option (B), It has planar structure and contains two different atoms C and O. One lone pair of sp3 hybridized atom O will participate in the conjugation , hence having 4e-. Hence, It is not a heterocyclic aromatic compound.
In option (C) and (D), It has non- planar structure containing sp3 hybridized atoms which will not participate in the conjugation. Hence, It is not a heterocyclic aromatic compound.
Hence, the correct answer is option (A).
Frequently Asked Questions-FAQs:
1. What are the uses of Heterocyclic compounds?
Answer: Application of Heterocyclic compound:
(i) Agrochemicals and pharmaceuticals both use heterocyclic compounds.
(ii) Heterocyclic compounds are used as starting materials in organic compound synthesis.
(iii) In corrosion inhibitors, sanitisers, anti-ordinates, and developers, heterocyclic compounds are used.
(iv) Pesticides, dyes, and plastics all contain heterocyclic compounds.
2. How stable are heterocyclic compounds?
Answer: Heterocyclic aromatic compounds with six members are more stable than those with five members. Six-membered rings are more stable than five and four-membered rings in alicyclic heterocyclic compounds.
3. What makes heterocyclic compounds biologically active?
Answer: Because of their activity in a variety of diseases, heterocyclic compounds are regarded as one of the most important classes of organic compounds used in a variety of biological fields. The heterocyclic ring is found in many biological molecules, including DNA and RNA, chlorophyll, hemoglobin, vitamins, and many others.
4. Why are heterocyclic compounds useful structures in medicinal chemistry?
Answer: Heterocycles are crucial for medicinal chemists because they allow them to expand the available drug-like chemical space and drive more effective drug discovery programmes.