-
Call Now
1800-102-2727
Halogenation of Alkynes - Addition Reactions, Halogenation, Dibromine addition, Hypohalous Acid Addition, Hydrogen Halides, Addition, Practice Problems and FAQs
You know well that alkynes contain at least one triple bond and opened to addition reactions across the triple bond. Do you remember, what happens when an alkene reacts with halogen?
The addition of halogens to an alkyne follows the same steps as the addition of halogens to alkenes not once but twice. Stepwise addition of halogen atoms to an alkyne molecule results in the formation of the corresponding dihalo alkene, which undergoes further reaction to form a tetra haloalkane. Similarly, hypohalous acid and hydrogen halides also reacts with an alkyne.
Let's discuss how these reactions takes place with a mechanism.
Table of Contents
- Addition reactions
- Halogenation to alkyne
- Addition of dibromine to propyne
- Addition of hypohalous acid to alkynes
- Addition of hydrogen halides to alkynes
- Practice Problems
- Frequently Asked Questions
Addition reactions
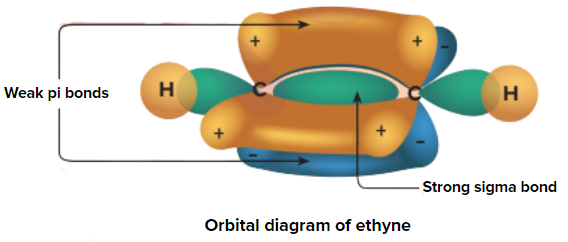
Due to the presence of two weak pi (π) bonds, alkynes undergo an addition reaction. Alkynes add up to two molecules of dihydrogen, halogens, hydrogen halides, etc.
An illustration of orbital overlap in alkynes representing the weak pi (π) electron cloud is given in
General addition reaction of alkynes
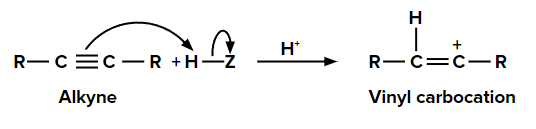
Let us consider the addition of any compound (HZ) to an alkyne.
Step 1: Addition of electrophile
When we add one equivalent of an electrophile (let’s say H+) to an alkyne, it forms a vinyl carbocation. This is an electrophilic addition reaction.
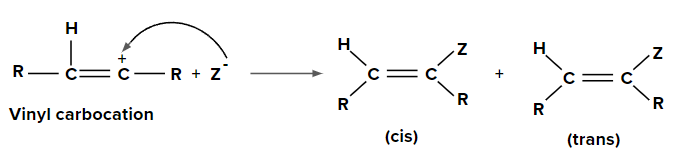
Step 2: Addition of nucleophile (Z-) to vinyl carbocation
Halogenation to alkyne
- Alkynes react with a molecule of X2 (in CCl4) to form 1,2-dihaloalkenes.
- Further, the reaction of one more molecule of X2 to 1,2-dihaloalkene produces tetra halogenated products.
-
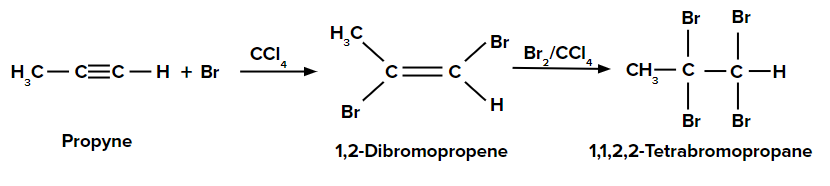
Addition of dibromine to propyne
When the first molecule of Br2 in CCl4 is added to propyne, an anti-addition reaction happens since the two substituents are added on the opposite sides of the pi bond.
Test for unsaturation with Br2 in CCl4
The reddish-orange colour of bromine solution in carbon tetrachloride is discharged when bromine adds up to an unsaturated site.
- Hence, this reaction is used as a test for unsaturation.
- Alkenes and alkynes decolourise the reddish-brown colour of Br2 / CCl4
We have hexane, hexene, and hexyne in three test tubes. When Br2 / CCl4 is added to hexane, the reddish brown colour of Br2 / CCl4 is retained. When Br2 / CCl4 is is added to hexene, the reddish-brown colour is discharged (decolourised) and 1,2-dibromohexane is formed. Similarly, when Br2 / CCl4 is added to hexyne, the reddish-brown colour is discharged (decolourised) and 1,1,2,2-tetrabromohexane is formed.


-
Addition of hypohalous acid to alkynes
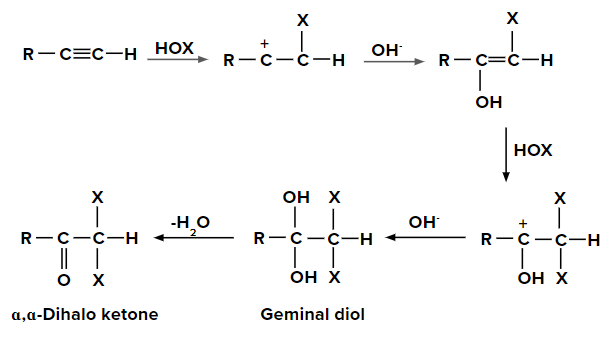
- Since the electronegativity of oxygen is higher than that of halogen except for fluorine, HOX breaks into OH- and X+.
- Two molecules of HOX (HOCl, HOBr or HOI) get added to alkynes and form geminal diols.
- This geminal diols form ⍺,⍺-dihalo ketones (two halogen groups are present at ⍺-position with respect to carbonyl group) on the elimination of H2O.
- The geminal diols are unstable. Therefore, they are easily converted to their respective ketones or aldehydes by dehydration (loss of one water molecule).
Generally, the -OH group is added to that carbon atom of alkyne, where more alkyl groups are present.
When HOX is added to the given alkyne, the pi electrons shift in such a way that the positive charge is present on the carbon attached to the alkyl (-R) group so that the positive charge is stabilized by +I effect of the alkyl group. When the second molecule of HOX is added to the alkene formed previously, the pi electrons shift in such a way that a positive charge is present on the carbon attached to the -OH group as the positive charge is stabilized by the +M effect of the -OH group.
-
Addition of hydrogen halides to alkynes
When two molecules of HX (HCl, HBr, or HI) are added to the alkynes, geminal dihalides (in which two halogen atoms are attached to the same carbon atom) is formed.
According to Markovnikov's rule, the addition of HX to unsymmetrical alkynes takes place.
Adding HBr to ethyne according to the Markovnikov rule
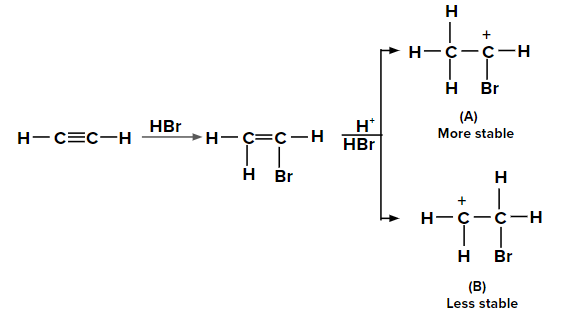
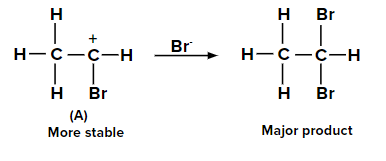
When the second molecule of HBr is added to bromoethene, two possible carbocations can be formed. The carbocation labeled as (A) is more stable than (B) due to the +M effect of the -Br group and also by the hyperconjugation of three ⍺-hydrogen atoms. Hence, the major product is obtained from carbocation (A).
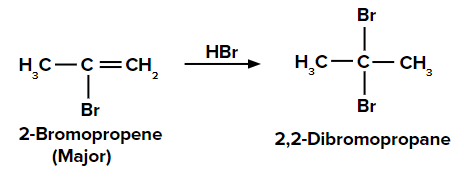
Adding HBr to propyne according to the Markovnikov rule
On adding the first molecule of HBr to propyne, we obtain 2-bromopropene and 1-bromopropene as the major and minor products, respectively due to the Markovnikov rule.
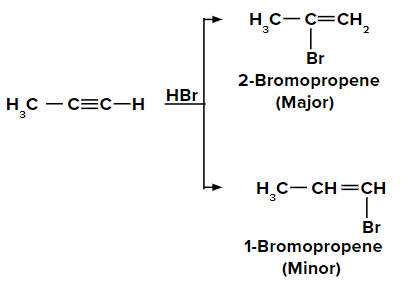
When the second molecule of HBr is added to 2-bromopropene, we get the final product as 2,2-dibromopropane.
Practice Problems
Q1. Alkynes undergo addition reactions as a result of
- Sigma electrons are tightly bound.
- There are sigma electrons that are loosely held.
- Pi electrons are tightly bound.
- Pi electrons are loosely held
Solution: Because of the presence of loosely held pi-electrons, alkynes undergo addition reactions. Because alkynes contain a triple bond, halogens, water, and other substances can be added to them via the addition reaction. A series of steps are followed to create addition products. The formation of addition products is caused by the stability of vinylic cations. Asymmetric alkynes follow Markovnikov's rule in order to undergo addition reaction.
So, the correct answer is an option (D).
Q2. When alkynes and halogens react, the following products are formed:
- Halogenated alkenes
- Halogenated alkanes
- Halogenated alkynes
- None of the preceding
Solutions: Alkynes and halogens undergo addition reaction to form halogenated alkenes which further react with halogens to give halogen-substituted alkanes.
So, the correct answer is an option (A).
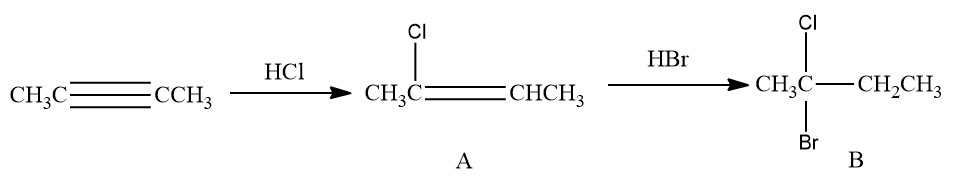
Q3. Predict product ‘C’ obtained in the following reaction of but-2-yne.
![]()
- 1-Bromo-1-chloro butane
- 2-Bromo-2-chloro butane
- 2-Bromo-3-chloro butane
- 3-Bromo-2-chloro butane
Solution: According to Markovnikov's rule, the addition of chloride ions will take place at the carbon having the lesser number of hydrogen. Hence, product A is 2-chloro butene.
Now, the addition of HBr to 2-chloro butene also takes place according to Markovnikov's rule and
forms 2-Bromo-2-chloro butane
Thus, option (B) is the correct answer.
Q4. Which of the following pairs can be distinguished by the Bromine water test?
- Propene and Propyne
- Pentane and Pentene
- n-Butane and Isobutane
- Hex-2-yne and Hex–3-yne
Solution: Bromine water is a test used for the detection of unsaturated hydrocarbons.
In option (A), both Propene and Propyne have unsaturation in their compound. Hence, they can’t be distinguished.
In option (B), Pentene has unsaturation whereas Pentane has no double bond in its structure. Hence, they can be distinguished and would be the correct answer.
In option (C), both n-Butane and Isobutane have no unsaturation in their compound. Hence, they can’t be distinguished.
In option (D), both Hex-2-yne and Hex-3-yne have unsaturation in their compound. Hence, they can’t be distinguished.
Hence, the Correct answer is an option (B).
Frequently Asked Questions
Q1. What is the function of the addition reaction?
Ans. Unsaturated compounds are added by a group of atoms or molecules to form saturated compounds in an addition reaction. It is widely used in the synthesis of various natural products and drugs. In industrial applications, vegetable oils are unsaturated compounds that are combined with hydrogen in the presence of a catalyst to form saturated compounds such as vegetable ghee.
Q2. What does halogenation imply?
Ans. Halogenation is a type of chemical reaction in which hydrogen atoms in a molecule are replaced by halogen atoms. The end product of halogenation is a compound with properties distinct from the starting compound.
Q3. Why is CCl4 used in halogenation?
Ans. Carbon tetrachloride is a non-polar inert solvent (CCl4). The primary goal of using these inert solvents is to dissolve the reactants present in the reaction. As an electrophile, Br+ attacks and undergoes electrophilic addition reaction on alkene. In fact, CCl4 has no effect on the reaction; it is simply used to distinguish it from the reaction in which H2O is the solvent, which results in the formation of a bromohydrin.
Q4. Why is sunlight required in halogenation?
Ans. These halogenations require energy input in the form of heat or light to begin. If light is used to initiate halogenation, each photon of light absorbed causes thousands of molecules to react. Halogenation reactions can take place in either a gaseous or liquid state.


