-
Call Now
1800-102-2727
Gram Atomic Mass: Atomic Mass, Molecular Mass, Molar Mass, Gram Atomic Mass, Gram Molecular Mass, Practice Problems & FAQs
Raushni, a student of class 11th went to a grocery store and asked for 2kg of sugar. The shopkeeper placed a 2kg weight on one side of the manual weighing scale and on the other side he started adding sugar till the weighing scale reached its equilibrium.

On her way back home Raushni came up with a question:
Is this exactly 2kg?
Well, if you look at the back side of weights, there is an authorized signature of the government. These weights have been calibrated according to the standard weight system. Hence, you can assure that the pulses are exactly 2kg.
A standard weighing system and a universally accepted unit are required to define any quantity. So numerous methods were established to define the mass of an atom such as molar mass, molecular mass, atomic mass, gram atomic mass & gram molecular mass.
In this concept page, we will understand the concept of gram atomic mass and how it came into inception!
TABLE OF CONTENT
- Atomic mass
- Molecular mass
- Mole Concept
- Molar mass
- Gram Atomic mass (G.A.M)
- Gram molecular mass (G.M.M)
- Practice problems
- Frequently asked question - FAQs
Atomic mass:
Atomic mass is defined as the amount of matter that makes up an element's atom. In other words, one atom's mass, measured in amu or u.
For the sake of numerical simplicity, we ignore the mass of the electron when calculating atomic mass (the mass of one atom) and assume that the mass of one proton is equivalent to one neutron's mass.
We can say,
(Total nucleons are equal to the sum of neutrons and protons.)
Example: Calculate the atomic mass of Na23 atom
Solution: number of proton = 11
Number of neutron = 23- 11= 12
Total number of nucleons = 23
Atomic mass of Na atom = 23 amu
Molecular mass:
Mass of a given molecule of a compound is referred to as molecular mass. In other words it can be also defined as the sum of the mass of all the atoms comprising the respective molecule is the molecular mass of the given molecule. It is generally measured in ‘amu’.
Example. Calculate the molecular mass of methane(CH4)
Solution:
Molecular mass of
|
Molecule |
Molecular mass |
|
N2 |
28 amu |
|
NH3 |
17 amu |
|
NaOH |
40 amu |
Mole Concept:
The amount of substance that contains the same number of fundamental components as the number of atoms present in a pure sample of carbon weighing exactly 12 g is known as a mole. A mole is the volume of a substance that contains exactly 6.0221023 of its elementary constituents.
one Mole= entities
number is known as Avogadro’s number and is represented by NA.
= 1 NA
|
Mole to number of entities |
Mole to mass |
Mole to Volume |
|
1 Mole= entities the number of moles = number of entities |
Number of moles = weight of substance (g)Molar mass The mass of particles of any substance are termed as the molar mass of the substance. |
Number of moles = volume of gas (L)Molar volume (L) (at same T & P) At STP, The number of moles = volume of gas (L)22.4 L (at STP) |
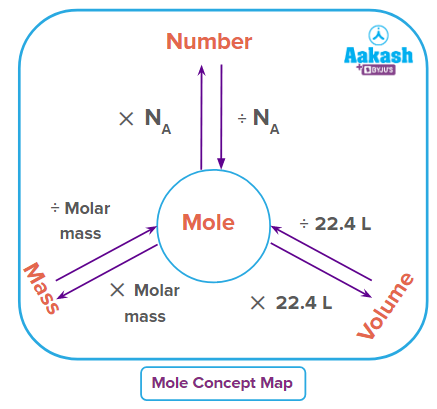
Molar mass:
A substance's molar mass is its mass in grams per mole of the compound.
For elements or molecules, the molar mass is displayed. The molar mass is simply the element's mass expressed in atomic mass units in the case of single elements or individual atoms. In other words, the atomic mass and molar mass of an atom are identical. Molar mass can be used to determine a particle's identity because it is equal to atomic mass for individual atoms. C, for instance, has an atomic mass of 12. Since carbon only contains one atom and is therefore found to be monatomic, its molar mass is also 12.
The mass of 6.0221023 entities of any particles are considered as the molar mass of that particular substance.
Molar mass is the weight of a single mole of an individual molecule, as opposed to molecular mass, which is the weight of a single molecule.
The molar mass of C atom = mass of C atom
Molar mass of NH3 molecule = mass of NH3 atom
Molar mass of SO42- ion = mass of SO42- ions
Example:
Let's say someone has a mole of sodium hydroxide. The individual would contain 6.0221023 sodium hydroxide molecules in that mole of sodium hydroxide, based on Avogadro's number.
1 mol NaOH = NaOH molecules
Sodium hydroxide molecule dissociates to give :
One mole of sodium hydroxide produces one mole of sodium ions and one mole of hydroxide ions in accordance with the dissociation described above. Consequently, we can also state that one mole of sodium hydroxide yields 6.0221023 of sodium ions and hydroxide ions.
Thus,
Gram Atomic mass (G.A.M):
The mass of one mole of an element's atoms is known as the gram atomic mass of that element. Although it has its unit in grammes, it is numerically equivalent to the value of the element's atomic mass unit. For instance, let’s calculate the gram atomic mass of nitrogen. It’s atomic mass is 14 amu.
From mole concept, we came to know that
Number of moles of a substance
In case of nitrogen,
Atomic mass = 14 amu
1 mole of nitrogen
Hence, the given mass of nitrogen = 14 g and this is the gram atomic mass of nitrogen.
In other words, Atomic mass of an atom expressed in grams is called gram atomic mass or simply we can say, the mass of 6.0221023 atoms (Avogadro's Number) is the gram atomic mass.
Example. Calculate the gram atomic mass of B10 atom.
Solution: In case of boron,
Atomic mass = 10 amu
1 mole of boron
Hence, the given mass of nitrogen = 10 g and this is the gram atomic mass of boron.
Note:
|
Element |
Relative Atomic Mass |
Atomic mass |
Gram atomic mass |
|
N |
14 |
14 amu |
14 g |
|
C |
12 |
12 amu |
12 g |
|
H |
1 |
1 amu |
1 g |
|
Ne |
20 |
20 amu |
20 g |
|
O |
16 |
16 amu |
16 g |
|
Na |
23 |
23 amu |
23 g |
Gram molecular mass (G.M.M):
A substance's molecular weight or the total of all the atomic masses in its molecular formula is equivalent to one gramme of molecular mass. It is equivalent to a molecule's molecular mass in grams. Mass of 1 molecule expressed in grams or mass of 6.0221023 molecules.
Example. . Calculate gram molecular mass of water (H2O)
Solution:
Molecular mass of H2O= (2 atomic mass of H atom) + (1 atomic mass of O atom)
= (2 1 amu) + (1 16 amu) = 18 amu
Gram molecular mass of H2O= (2 GAM of H atom) + (1 GAM of O atom)
= (2 1 g) + (1 16 g) = 18 g
|
Molecule |
Gram Molecular mass |
|
CO2 |
44 g |
|
KOH |
56 g |
|
NaCl |
58.5 g |
Recommended Video: Introduction to Atomic and Molecular Weight: Mole Concept Class 11 Chemistry (Concepts) | JEE 2024
Practice problems:
Q 1. Which of the following represents the gram molecular mass of CO2.
- 44 g
- 18 g
- 4.4 g
- 56 g
Answer: (A)
Solution: Molecular mass of CO2= (1 atomic mass of C atom) + (2 atomic mass of O atom)
Gram molecular mass of CO2= (1 GAM of C atom) + (2 GAM of O atom)
Q 2. Calculate the gram atomic mass of Mg.
- 14 g
- 24 g
- 44 g
- 34 g
Answer: (B)
Solution: In case of Magnesium,
Atomic mass = 24 amu
1 mole of Magnesium
Hence, the given mass of Magnesium= 24 g and this is the gram atomic mass of Magnesium.
Q 3. Find the total mass in grams of 6 moles of Na ? (Molar mass of Na= 23 g mol-1)
- 138 g
- 158 g
- 238 g
- 13.8 g
Answer: (A)
Solution: number of moles =
Q 4. Which of the following is the correct unit gram atomic mass?
- g/litre
- g/mol
- g
- None of the above
Answer: (C)
The mass of one mole of an element's atoms is known as the gram atomic mass of that element. Gram atomic mass is measured in grams. Hence, option C is the correct choice.
Frequently asked question-FAQs:
1. Can any element's relative atomic mass be fractional?
Answer: Because it is just the total number of protons and neutrons, relative atomic mass cannot ever be expressed as a fraction. There are never any fractions of protons or neutrons.
2. Is it possible to define relative atomic mass in relation to elements other than carbon?
Answer: Yes, relative atomic mass is defined mathematically in relation to any other element, but in the scientific community, carbon is regarded as the standard.
Standard definition; Relative atomic mass =
In respect of oxygen; Relative atomic mass =
3. Why is the mass of Chlorine written in fraction?
Answer: Cl35.5 written because of its isotopes, Cl present in nature in different isotopes in different natural abundance (%). So, due to their isotopes, they have fractional mass. We can call it weighted average atomic mass.
4. What distinguishes atomic mass from atomic weight?
Answer: Atomic weight is typically the weighted average of isotopic abundance in nature as opposed to atomic mass, which is typically used for single isotopes. Because it is correlated with the number of protons and neutrons in the nucleus, atomic mass is always a whole number.


