-
Call Now
1800-102-2727
Gibbs Helmholtz Equations: Gibbs Free Energy, Gibbs Helmholtz Equation Derivation, Applications, Practice Problems, FAQs
Consider a person riding a cycle. Initially, he will be having more energy and hence pedalling fast and covering more distance in a unit of time. The rate of distance covered starts decreasing with the distance he has cycled. His cycling capacity after covering 5Km will be less than at starting speed and still less after covering 10kms ie there will be a deceleration in his speed.
Like a cycler, any system or its contents possesses some energy(internal energy or enthalpy) and entropy(freedom of movement). The ability of the system to do some work is given by a term called free energy. Depending on the conditions of work, we have two free energy. Helmholtz free energy (A) and Gibb’s free energy (G). Gibbs's free energy quantifies the work the system is capable of doing at constant temperature and at constant pressure. This will be the maximum work other than the work of expansion (in the case of gases) we can make the system do. It is a thermodynamic property that characterises the system in terms of its energy and entropy at a specified temperature and is a state function.
A system undergoes changes in its free energy on changing temperature like a cycler riding some distance. Within narrow temperature changes, the change in free energy with temperature can be considered linear or proportional. But, the free energy changes for the same change in temperature, differ when compared between a wide range of temperatures.
Consider two systems-one at 30°C and another at 40°C. The two systems have different free energies. No, let us increase the temperature of each system by 10°C. In spite of the same increase in temperature, The two systems will not get the same free energy increase. The increase in free energy of the first system will not be equal to the free energy increase in the second system. The free energy changes for the same temperature are not equal but depend on the temperature of the system. So, the maximum work that the system can do also does not increase equally.
This maximum work that a system can do depends on the temperature of the system. It changes at different temperatures as the cycler's capacity changes with the distance he covers.
Like the cycler, the ability to do work by the system decreases in higher temperature ranges.
Gibbs Helmholtz equation gives a quantitative estimation of the change in the Gibbs free energy with temperature.
In the following text, you will know more about free energy and the effect of temperature on free energy changes.
Table of Content
- Gibbs Free Energy
- Derivation of Gibbs Helmholtz Equation
- Applications of Gibbs Helmholtz Equation
- Practice Problems
- Frequently Asked Questions- FAQs
Gibbs Free Energy
The maximum work that a system can perform at constant temperature and pressure is called free energy or Gibb’s free energy. This is the useful work other than work of expansion or compression available within the system and equal to the difference between the enthalpy of the system and the heat energy changes accompanying the entropy of the system
Mathematically, Gibb’s free energy is given as
G = H – TS
Where
G is the Gibbs free energy
H is the enthalpy of the system
T is the temperature of the system and
S is the entropy of the system
In terms of internal energy (U) of the system, it can also be written as-
G = U + PV – TS
Where,
- U is the internal energy
- P is the pressure
- V is the volume
- T is the temperature and
- S is the entropy of the system.
Free energy Changes
A system changes thermodynamically, it free energy also changes. But, Gibbs free energy is a state function and doesn’t depend on the path used for reaching the final state from the initial state. So, the change in Gibbs free energy will be also in terms of the change in the enthalpy and entropy of the system due to the difference in the temperature of the system.
The change in the free energy of the system between the initial and final states of the system can be given as-
Gf - Gi = ΔG = ΔH – Δ(TS), where, Gf is the free energy of the initial state and Gi
Is the free energy of the final state.
If the reaction is carried out under constant temperature{ΔT=O}
Change in free energy = ΔG = ΔH – TΔS
This equation is called the Gibbs equation.
This equation is important because it stipulates the conditions of the feasibility of the change it has to undergo.
If the free energy of the initial state is higher than the final state, then the free energy change is negative, or ΔG < 0; the reaction will be spontaneous and exothermic.
If the free energy of the initial state is lower than the final state, then the free energy change is positive, or ΔG > 0; the reaction will be nonspontaneous and endothermic
If the free energy of the initial state is the same as the final state, then the free energy change is zero or
ΔG = 0; the reaction is at equilibrium
Note:
- ∆G serves as the single master variable that determines whether a given chemical change is thermodynamically possible. If ΔG is negative, the process will occur spontaneously and is referred to as exergonic.
- ΔG determines the direction and extent of any process or chemical change.
- Therefore spontaneity is dependent on the temperature of the system.
Derivation of Gibbs Helmholtz Equation
Gibb’s Helmholtz equation gives the dependence of free energy variation with respect to the temperature.
Let G be the free energy at temperature T. We like to know the changes in this (GT) with T.
To find the variation of the free energy (G) at a temperature (T) with temperature, we shall difference (GT) with respect to T ie (GT)T
The differentiation can be done by the quotient rule as-
We have to find the variation of free energy with temperature ie .
For this consider the Gibbs free energy definition-
G = H -TS
Differentiating on both sides,
dG = dH - TdS - SdT
At constant T and P conditions,
dG = dH - TdS…..(2)
Enthalpy H = U + PV, where U is the internal energy. P and V are the pressure and volume of the system.
dH = dU + PdV + VdP……(3)
Also, as per the first law of thermodynamics, U = Q + W, where Q and W are the heat and work related to the system with appropriate signs with respect to the system.
dU = dQ - dW…….(4)
And, S = QT, or Q = TS, where S is the entropy of the system. And dQ = TdS + SdT
W = PV considering only the work of expansion done by the system or compression done on the system.
dW = PdV + VdP
Substituting, for dQ and dW in equation 4,
dU = TdS + SdT - PdV - VdP
At constant T and P conditions,
dU = TdS - PdV…(5)
Substituting dU from equation (5) in equation (3)
dH = TdS - PdV + PdV + VdP
dH = TdS + VdP…..(6)
Substituting the value of dH from (6) in equation (2),
dG = TdS + VdP - TdS - SdT
dG = VdP - SdT
At constant pressure condoitions,
dG = - SdT or - S = GT…… (7)
Substitute the value of GT in the equation (1),
But, G = H -TS or G + TS = H
Substituting for TS + G,
In standard states
These relations gives the variation of the free energy with temperature and called as
Gibb’s Helmholtz equations.
The equation can also be written as-
In standard states,
This is also another form of Gibb’s Helmholtz equations
Applications of Gibb’s Helmholtz Equation:
- Calculating enthalpy of exothermic or endothermicity of reactions
Gibb’s Helmholtz equation of free energy changes with temperature is
Gibb’s Helmholtz equation for free energy changes between states with temperature can be written on the same lines as-
Gibbs Helmholtz equation for standard free energy changes between states with the temperature at one bar pressure can be written on the same lines as-
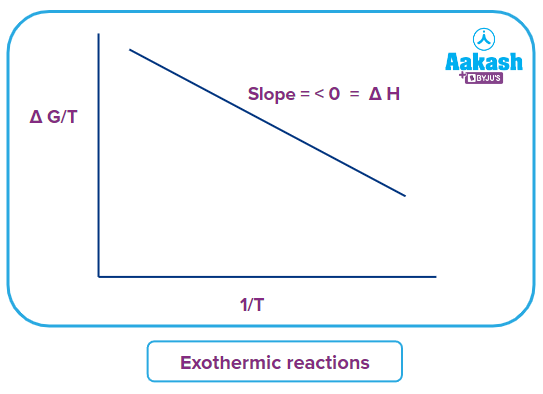
A plot of (G0T) against (1T) will be a straight line with a slope of of H.
Slope of the line will be able to differentiate exothermic and endothermic reactions.
For exothermic reactions, Tfinal > Tinitial and Gfinal > Ginitial
The plot for an exothermic reaction will have negative slope.
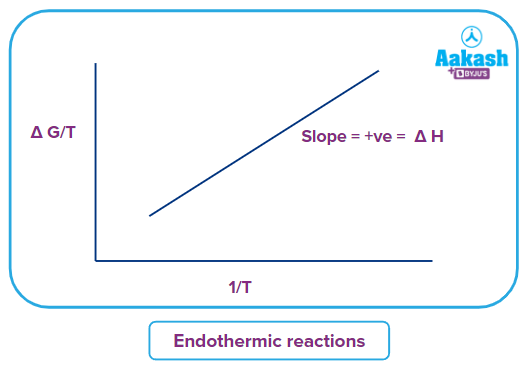
For endothermic reactions,
Tfinal < Tinitial and Gfinal < Ginitial
And the slope will be positive.
- To calculate the standard Free energy changes,
Gibbs Helmholtz equation is-
Integrating the equation to find the changes between two states of the system or reactants and products of a reaction,
Gf0T2 - Gi0T1 = H0 {1T2 - 1T1}
Knowing the standard free energy of formation at one temperature and assuming the constancy of the known enthalpy, we can calculate standard free energy of the reaction at another temperature.
- To calculate the entropy of the reactions.
Calculation of standard enthalpy change of the reaction can be used to calculate the entropy of the substance at that temperature as,
- The standard free energy estimation can be used to calculate the standard EMF of a cell and equilibrium consonant of reactions as well from the relations-
, where E0 is the standard EMF of a cell.
Practice Problems
(1) Calculate the temperature at which the formation of ammonia from hydrogen and nitrogen will be spontaneous.
N2(g) + 3 H2(g) ⇔2 NH3(g).
Enthalpy and entropy of the reaction are 92kJmol-1 and 200JK-1mol-1 respectively.
Solution:
Reaction will be spontaneous when or
Substituting the values of entropy and enthalpy,
The formation of ammonia from its elements will become spontaneous at temperatures higher than 460K.
(2) The standard free energy change of a reaction is 50kJ and 70kJ at temperatures 37°C and 47°C respectively. The standard enthalpy of the reaction is?
a. 580kJ
b. 60kJ
c. -580kJ
d. -60kJ
Answer: Option C
Temperature in K are, 37°C = 310K and 47°C = 320K.
Gibbs Helmholtz reaction says,
Substituting the given values,
0.219 - 0.161 = H0 ( 0.0031 - 0.0032)
0.058 = -0.0001H0
(3) Predict whether the following reaction is spontaneous or not at 25°C?
N2(g) + 3 H2(g) ⇔ 2 NH3(g
∆H = -92.22 kJ
∆S= -198.75 J/K
Solution:
∆H is favourable
∆S is unfavourable
Before we can compare these terms to see which is larger, we have to incorporate into our calculation the temperature at which the reaction is run:
T(K) = 25° C + 273.15 = 298.15 K
We then multiply the entropy of reaction by the absolute temperature and subtract the T S term from the H term:
∆G° = ∆H° – T∆S°
= -92,220 J – (298.15 K x -198.75 J/K)
= -92,220 J + 59,260 J
= -32,960 J
∆G°= -32.96 kJ
4. If the slope of the plot of the temperature coefficient of Gibbs free energy against the reciprocal of temperature is positive the reaction is
a. Exothermic
b. Endothermic
c. Insufficient data
d. Cannot be determined from the slope
Answer: Option B
In the plot of the change in the Gibbs free energy against reciprocal temperature, a positive slope indicates an endothermic reaction
Frequently Asked Questions (FAQs)
1. What is the difference between Gibbs's free energy and Helmholtz's free energy equations?
Solution: Gibbs's free energy equation gives the maximum available useful work ( not including the pressure-volume work) from the system. Helmholtz free energy represents the total work including the pressure-volume work available from the system
2. What is the difference between the Gibbs free energy equation and Gibbs Helmholtz equations?
Solution: Gibbs's free energy equation represents the useful work capacity of a system, while the Gibbs Helmholtz equation gives the variation of this useful work of the system with temperature.
3. What is the temperature coefficient of free energy?
Solution: The change in the free energy of a system with changes in the temperature of the system is called the temperature coefficient of free energy. It is represented as GT
4. Can you estimate the enthalpy of a reaction from the temperature coefficient?
Solution: Yes. If the ratio of the free energy to temperature is positive the reaction is endothermic and if negative the reaction is exothermic. The magnitude gives the enthalpy change of the reaction.


