-
Call Now
1800-102-2727
General Organic Chemistry – Reaction Intermediates, Electronic Effects, Applications, Practice Problems and FAQ
Organic chemistry is no less than a factual art. Learning any new art requires passion and interest. And this particular topic is full of innumerable thrilling facts and is so much around us, that if given a chance, every human soul can fall in love with this art. And of course, a little practice can make you a pro at it!
The study of carbon-containing molecules' structure, characteristics, content, reactions, and synthesis is known as organic chemistry. The majority of organic compounds are composed of carbon and hydrogen, though they can also contain a variety of additional elements like nitrogen, oxygen, halogens, phosphorus, silicon, and sulphur.
Things around us are a major component of organic compounds. A few common examples are shown in the below image.

Understanding chemical molecules and their functioning will help us comprehend how living organisms function. Given how significant life is to us as humans, it is probably not a surprise that organic chemistry has many applications in daily life.
Let us learn more about the mechanistic approach to organic chemistry!
TABLE OF CONTENTS
- General Organic Chemistry – Introduction
- Reaction Intermediates in Organic Chemistry
- Electronic Displacement Effects
- Practice Problems
- Frequently Asked Questions – FAQ
General Organic Chemistry – Introduction
Carbon is an element that forms powerful chemical bonds with other carbon atoms as well as many other elements like hydrogen, oxygen, nitrogen, and halogens, is the subject of organic chemistry. There are more than a million known carbon compounds due to the ability of carbon in creating covalent bonds.
The major step followed in any organic reaction is discussed below:
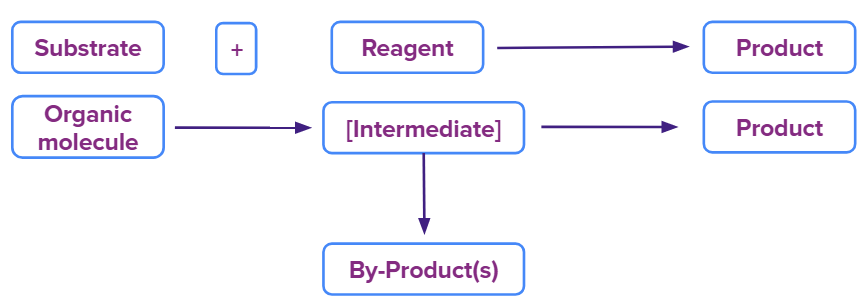
Reaction Intermediates in Organic Chemistry
The intermediate that is created when covalent bonds break is a fragile and highly reactive fragment.
The three types of intermediates that we encounter most frequently in various organic reactions are carbanions, free radicals, and carbocations.
Carbocation
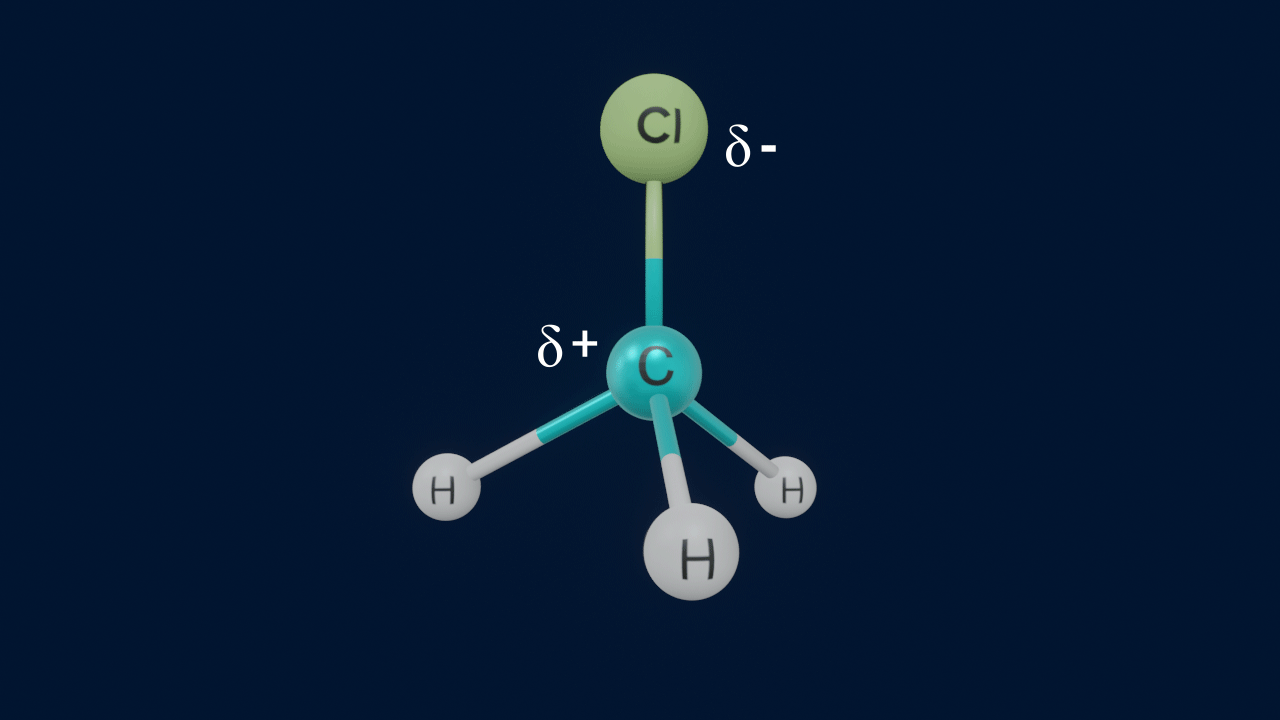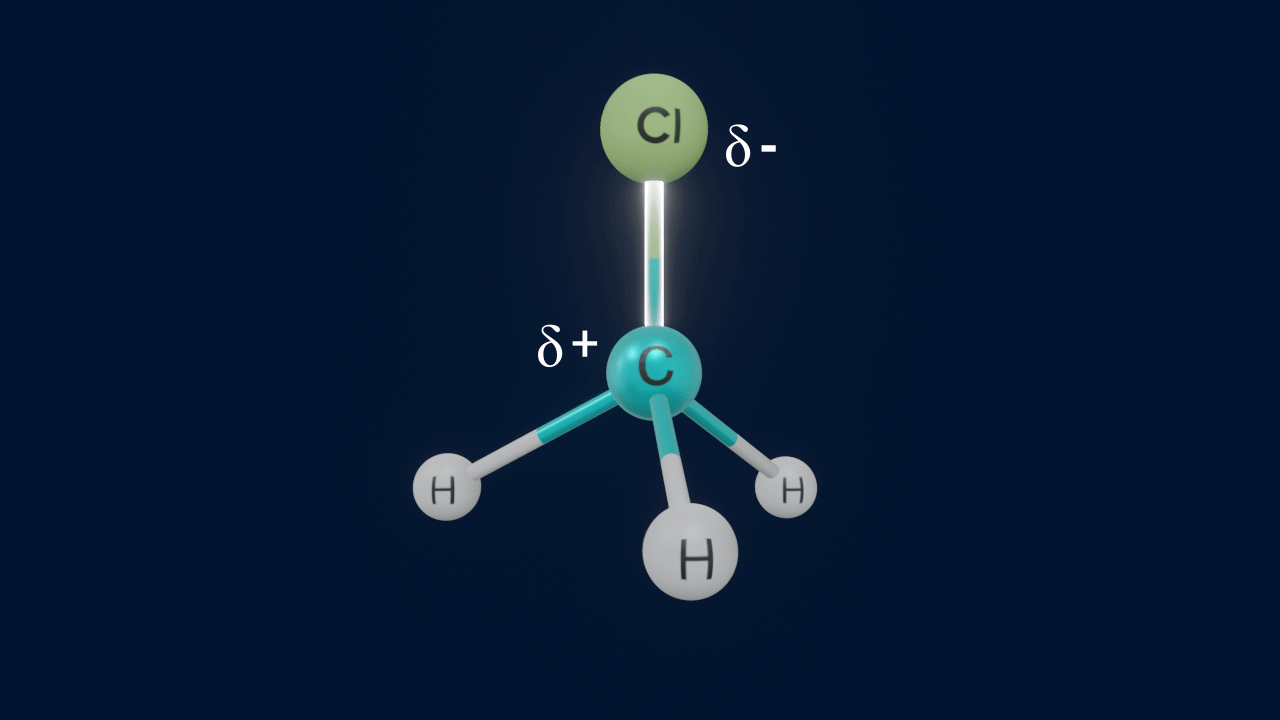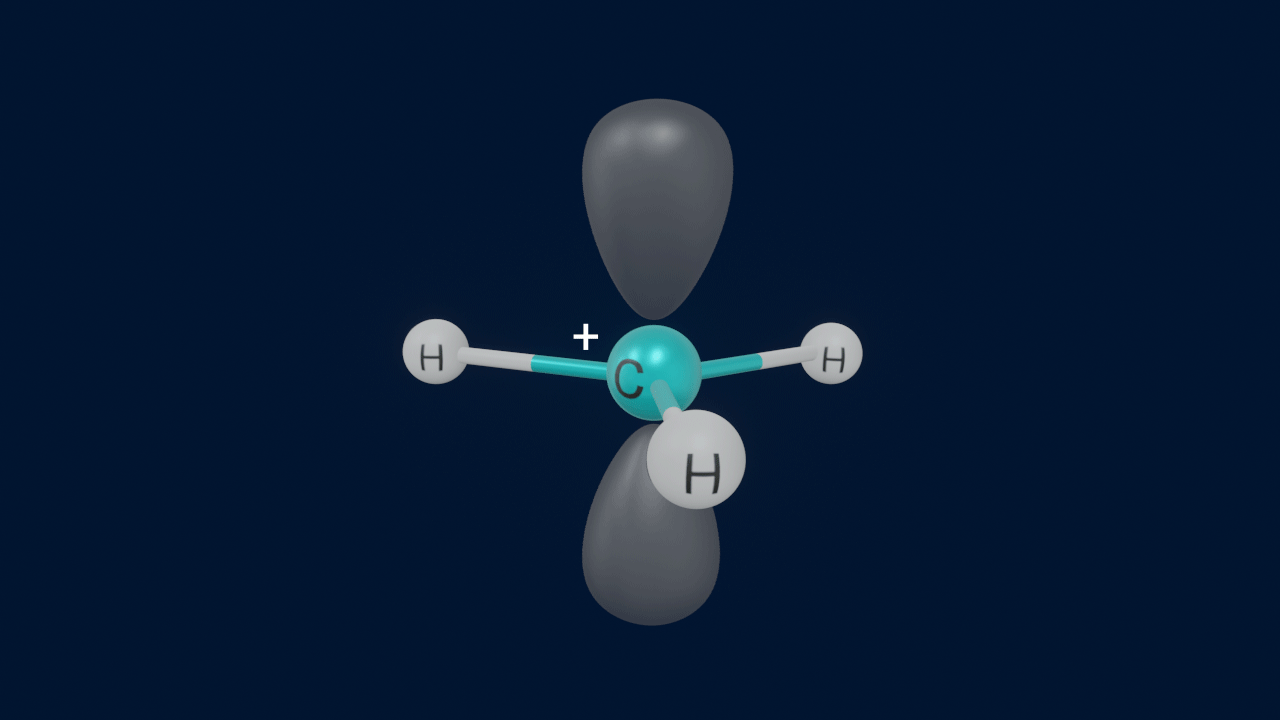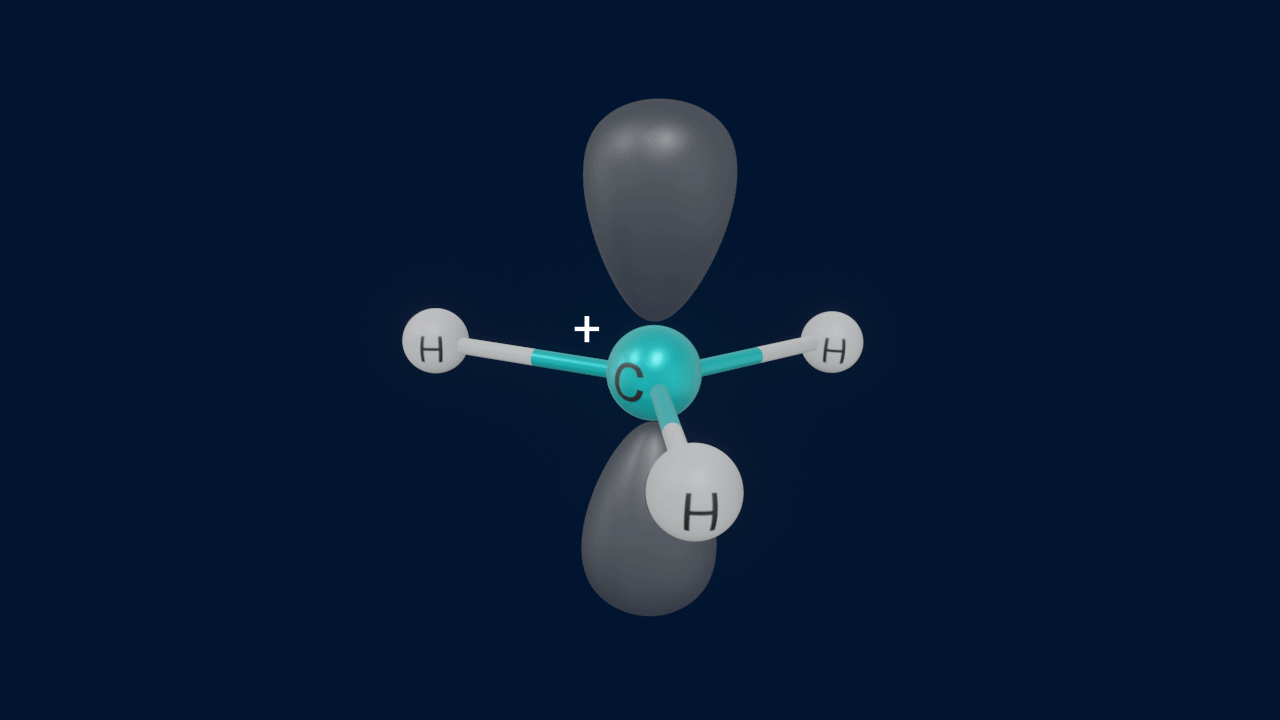
When a covalent bond between carbon and an electronegative counterpart disintegrates by heterolytic fission, the atom that is more electronegative abstracts the electron pair, whereas the carbon loses one electron and gains a positive charge. Carbocations are organic compounds with one atom of carbon with a positive charge.
Important points to remember about carbocations
- Carbon has a positive charge and possesses six electrons.
- Steric number (S.N.) = 3 of the carbocation indicates that the carbon atom is sp2 hybridised.
- Shape of the carbocation is trigonal planar.
- The nature of carbocation is electron-deficient due to its sextet configuration (6e-) and unoccupied p-orbital.
Carbanion
When a covalent bond between carbon and a comparatively less electronegative counterpart disintegrates by heterolytic fission, the carbon atom abstracts the electron pair, whereas the less electronegative counterpart loses one electron. Therefore, the carbon atom gains a negative charge.
Important points to remember about carbanions
- Eight electrons i.e complete octet and a negative charge are present on carbon in a carbanion.
- Steric number (S.N) of the carbanion is 4 which indicates that the carbon atom is sp3 hybridised.
- Shape of the carbanion is trigonal pyramidal.
Carbon Free Radical
When carbon has seven electrons in its valence shell, it forms a peculiar species known as a carbon-free radical. The homolytic breakage of covalent bonds results in the generation of free radicals.
Important points to remember about carbon free radicals
- The total number of electrons in an atom of carbon in a carbon-free radical is seven.
- In the carbon free radical, the carbon atom is sp2 hybridised.
- The shape of the carbon-free radical is trigonal planar.
Electronic Displacement Effects
- The electronic displacement effects happen when shared electrons in an organic molecule are moved from one atom to another atom.
- The presence of an atom or group in the molecule or the presence of an attacking reagent are both potential causes of electronic displacements in covalent bonds.
- This displacement is dependent on both internal and external elements that may exist within the molecule. Based on this, there are two forms of electrical displacement effect, which are detailed below.
Inductive Effect
It is the permanent polarisation or displacement of a bonded pair of sigma (σ) electrons towards the more electronegative atoms/substituents. Electronegative atoms have the ability to attract the shared pair of electrons towards themselves. Thus, they attract the sigma bonded electrons, which leads to the polarisation of the bond.
Characteristics of Inductive Effect
- Displacement of the electrons is due to the electronegativity difference between the atoms.
- The displacement towards the more electronegative atom / group is a permanent electronic effect.
- The displacement causes the permanent polarisation of bonded electrons.
- It operates through σ bonds only, i.e., no displacement of π electrons takes place.
- The electrons never leave their original orbitals. Only displacement takes place, but the electron
remains in its orbitals itself.
- It is a distance-dependent effect. As the distance increases, the strength of the inductive effect decreases. Generally, it is effective up to the third carbon. Its effect becomes negligible and insignificant after the third carbon bond.
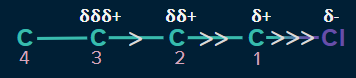
Types of Inductive Effect
There are two types of Inductive effect, namely
- + I effect
- - I effect
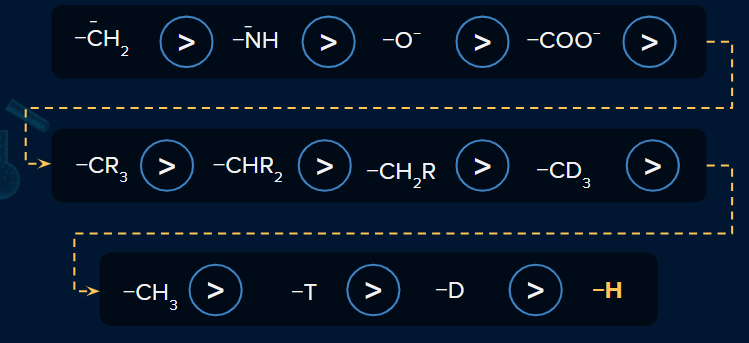
+I Effect
The permanent shift in the electron density along the carbon chain away from the electron-donating group (EDG) is known as the +I effect.
Order of +I Effect
–I Effect
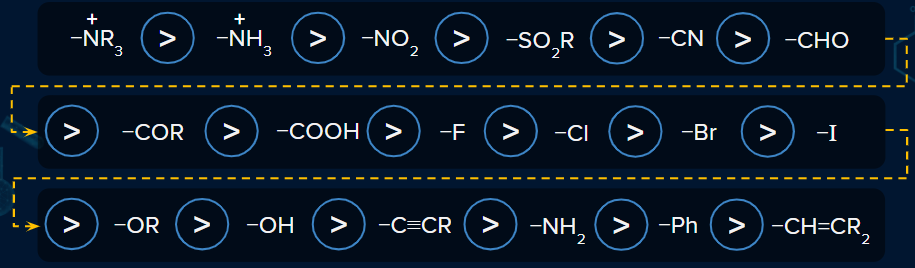
The permanent shift in the electron density away from the electron-donating group (EDG) or along the carbon chain towards the electron-withdrawing Group (EWG) is known as the -I effect. Groups which show -I effects are -COOH, -Cl, -F, -NO2, -CN, etc.
Order of -I Effect
NOTE
The electron-donating/withdrawing capability is relative to hydrogen. The inductive effect of hydrogen is considered to be zero.
Applications of Inductive Effect
- The relative stability of intermediates can be determined using the inductive effect.
- Groups showing -I effect stabilise electron-rich species and destabilise electron-deficient species. Electron withdrawing groups (due to -I effect) will nullify the negative charge on the negatively charged species and hence, will stabilise the negatively charged species. Whereas, an electron-donating group (due to +I effect) will destabilise a negatively charged species.
- Groups showing +I effect stabilise the electron-deficient species and destabilise the electron-rich species. Electron donating groups (due to +I effect) will intensify the negative charge on the negatively charged species and hence, will destabilise the negatively charged species. Whereas, an electron-withdrawing group (due to -I effect) will stabilise a negatively charged species.
Resonance or Mesomeric Effect
The delocalisation of high-energy electrons ( electrons or lone pair of electrons) takes place in this effect. In the case of resonance, several alternative Lewis structures—rather than just one—are employed to represent the bonding and characteristics of a given molecule.
Numerous Lewis structures that together explain the delocalisation of electrons in a molecule are known as resonating structures, and they can all be drawn for a molecule.
Let’s take the example of CO3-2.
Conditions for Resonance
- The atoms which are involved in conjugation must be in the same plane.
- There should be at least three continuous parallel p-orbitals (or d-orbitals) on adjacent atoms.
- The parallel p-orbitals or d-orbitals can be half-filled, fully filled, or vacant.
Types of Resonance or mesomeric Effect
Mesomeric effect is further divided into two categories, namely
- +R effect (positive resonance effect)
- -R effect (negative resonance effect)
+R or +M Effect
- In this effect, an atom or substituent group connected to the conjugated system transfers electron density towards that system. Due to this electron displacement, some individual locations have large electron densities.
- These conjugate complexes react more favourably with electrophiles and less favourably with nucleophiles.
- It is important to remember that for the group to exhibit the +M effect, there must be either a lone pair of electrons or a negative charge.
- Groups showing +M or +R effects are mentioned here. Here. ‘R’ mentioned in the group is considered an alkyl group.
- If we take any group ‘G’ having a lone pair or an extra electron like an anion, then below are the possible cases. G can be any of these groups like -NH2,-OH,-Cl,-OR etc.
Order of +R or +M Effect
There are many groups having lone pairs or negative charges that when attached to a conjugated system show +R or +M effect, but it is difficult to decide which is showing more +R or +M effect.
Based on this order, we can easily decide which group has more +R or +M effect.
-R or -M Effect
- When 𝜋-bond electrons are pulled off from the conjugate system to a particular group, the conjugate system's electron density decreases, which causes the negative mesomeric (-R or -M) effect.
- It is important to remember that for the -R or -M effect to occur, the group must either have a positive charge or a vacant orbital.
- By reducing the electron density in the conjugate system, the -R or -M effect increases a molecule's reactivity toward nucleophiles while decreasing its reactivity toward electrophiles for the same reasons.
- Groups showing -R or -M effect are
Order of -R or -M Effect
Based on this order we can easily decide which group has more -M effect.
Applications of Resonance or Mesomeric Effect
The following are some of the mesomeric effect's numerous applications.
The Stability of Carbocation
One use of the mesomeric effect is to enhance the stability of the carbocation. All aromatic compounds are always more stable than non-aromatic compounds due to the influence of resonance. The more the conjugation or more the number of resonating structures, the greater will be the stability.
- It has been discovered that the carbocation in which the positive charge is conjugated with a double bond is more stable. Delocalisation of π-electrons of conjugated double bonds results in resonance structures, which leads to increased stability. For Example, the stability of allylic carbocation exceeds that corresponding alkyl cation due to the resonance. When there is true conjugation between the vacant p orbital and adjacent lone pair or electrons, strong stability is created.
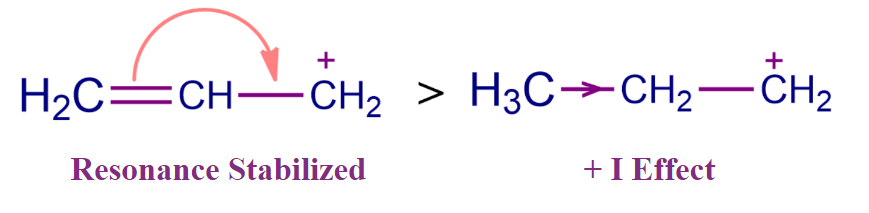
The Stability of Carbanion
The stability of the carbanion is increased by the presence of double bonds or an aromatic ring close to a negatively charged carbon atom due to resonance.
For example, because of resonance, the negative charge on the benzyl carbanion was distributed over other carbon atoms, making it more stable than the ethyl carbanion.
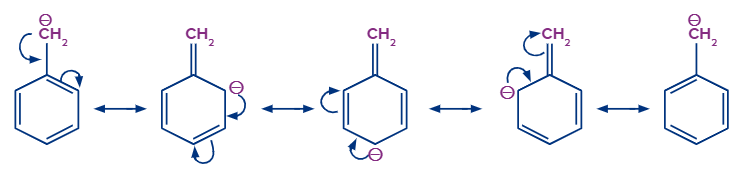
Stability of Free Radical
Simple alkyl radicals are less stable than allylic and benzylic types of free radicals because of the delocalisation of the unpaired electrons throughout the system.
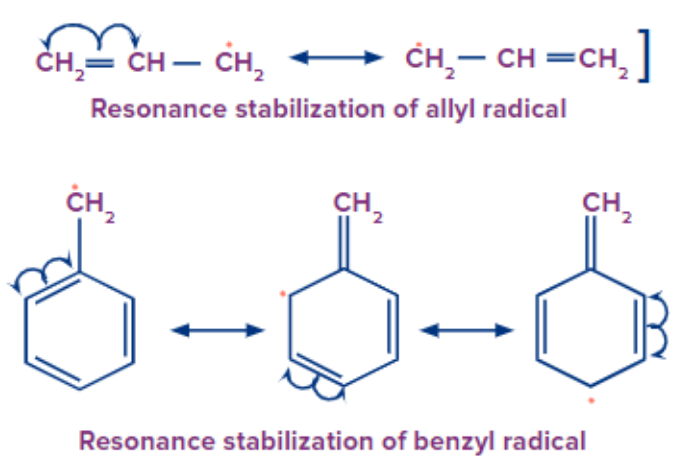
Acidic and Basic strength
- The most prominent factor in determining the strength of an acid is the stability of its conjugate base—the more stable the conjugate base is, the stronger the acid will be.
- Based on electron pair availability, basicity may be seen in a practical manner. The easier it is for electrons to be transferred to create a new bond, the stronger the base will be.
- After the loss of a proton, if the conjugated system forms a stable conjugate base, then it is more acidic than a non-conjugated system. The mesomeric effect helps in deciding the acidic and basic nature of the system.
Example 1: When two compounds are showing mesomeric effects, then we should consider a few points.
Acidic strength ∝ -M group
Basic strength ∝ +M group
Let us understand a few important cases.
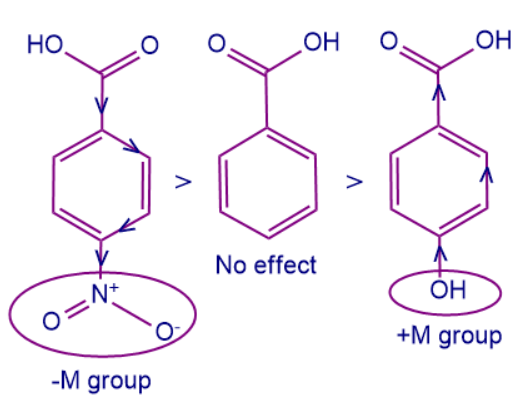
Case 1: Let us consider the below-mentioned system, and we have to arrange the molecules based on their acidic strength.
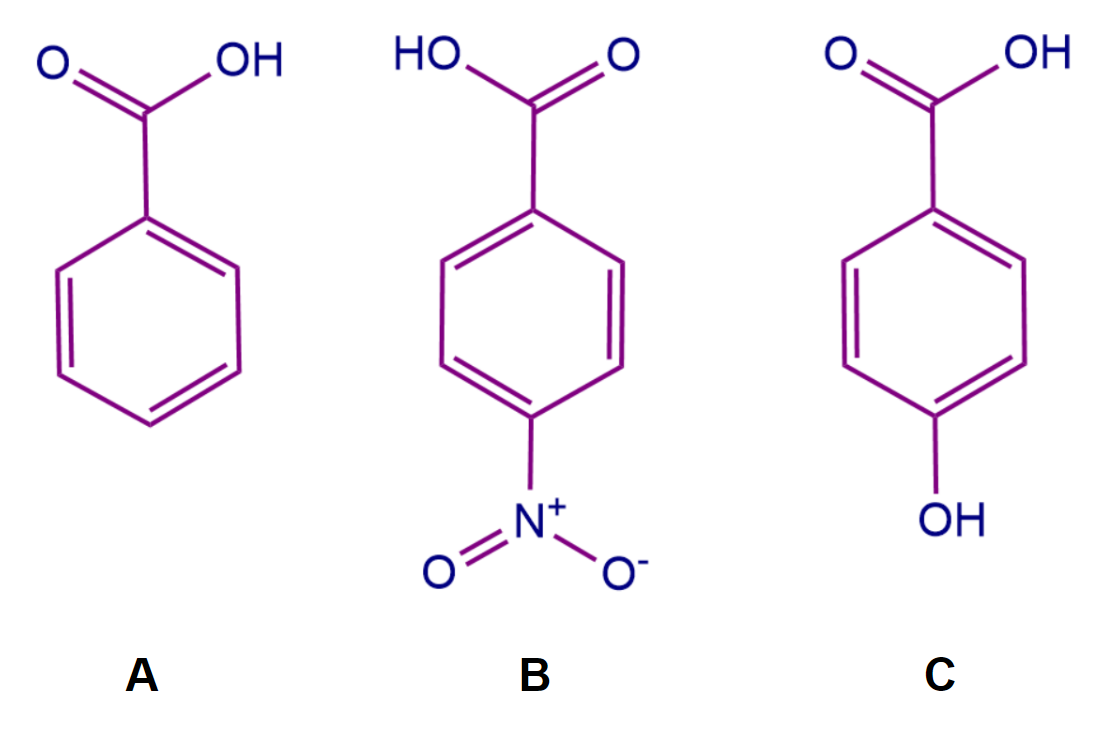
Here ‘A’ is benzoic acid, whereas ‘B’ and ‘C’ are derivatives of benzoic acid. To arrange the acidic strength we should remember that -M group attached to the conjugated system withdraws more electron density and it is easy to remove protons from the conjugated system. Whereas +M group increases the electron density making it difficult to remove the proton, thus decreasing the acid strength of the system. So, the correct order of acidic strengths is B>A>C.
Hyperconjugation
The delocalisation of σ electrons of a C-H bond with a nearby vacant p-orbital or an antibonding π* orbital in an unsaturated or electron-deficient system is known as the hyperconjugation effect. It is also known as no bond resonance, Baker–Nathan effect, or σ-π conjugation.
Hyperconjugation is a permanent effect. It results in increased instability due to resonance-type stabilisation.
Conditions for Hyperconjugation
- At least one α-hydrogen has to be present at the saturated α-carbon (sp3 hybridised) with respect to alkenes, benzene, carbocation, and free radicals.
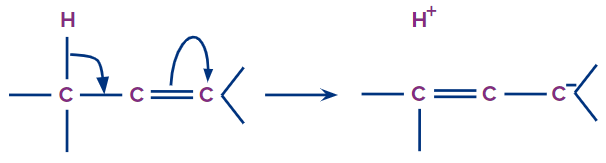
- More the number of α-hydrogens (hydrogens present on the α- carbon atom), the more the number of resonance structures, and the higher the stability due to hyperconjugation
- A vacant p-orbital or an antibonding π* orbital, will be present on the atom adjacent to the α- carbon atom.
Application of Hyperconjugation Effect
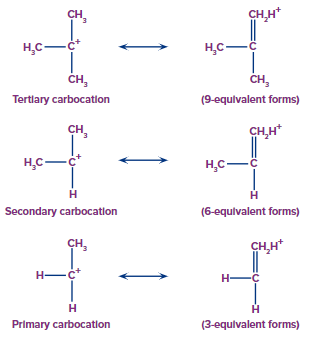
Stability of Carbocation
The stability of carbocation depends on the hyperconjugation effect. As the number of α-hydrogens increases, more resonating structures are possible by hyperconjugation and the stability of the intermediate increases.
Tertiary butyl carbocation > Isopropyl carbocation > Ethyl carbocation > Methyl carbocation
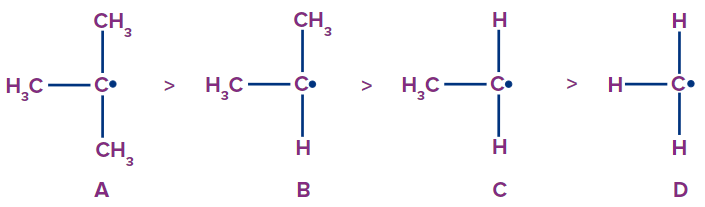
Stability of Free Radicals
The stability of free radicals depends on the hyperconjugation effect. As the number of α-hydrogens increases, the stability increases. It is the same as the stability of carbocations
Stability of Alkenes
The stability of alkenes depends on the hyperconjugation effect. As the number of α-hydrogens increases, the stability increases.
Stability of alkenes:
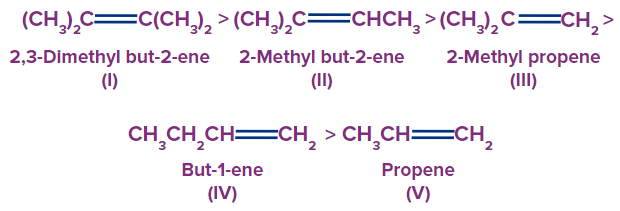
This order of stability is because of a greater number of contributing structures, causing larger delocalisation and hence the stability of alkenes.
Heat of Hydrogenation
Heat of hydrogenation is the amount of heat evolved/mol in the addition of hydrogen to form a saturated hydrocarbon.
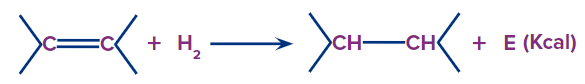
Heats of hydrogenation show that the greater the number of alkyl groups attached to the doubly bonded carbon atoms, the greater the stability (i.e., lower is the heat of hydrogenation) of the alkene or the lesser the heat of hydrogenation, the lesser the internal energy and more is the stability of the system. Hyperconjugation decreases the heat of hydrogenation.
The magnitude of Heat of Hydrogenation is as follows:
Example: cis-2-butene and trans-2-butene
The reaction for hydrogenation and energy profile diagram for cis-2-butene and trans-2-butene is
shown as follows.
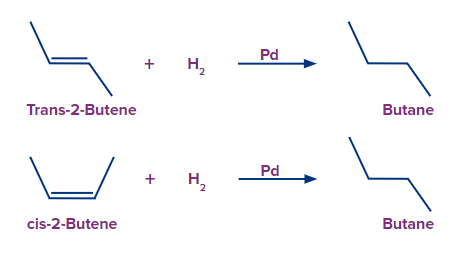
According to the reaction profile, the stability of alkenes increases, and the energy difference between an alkene and the product, i.e., the heat of hydrogenation decreases. Thus, the trans alkene releases less heat as compared to the cis alkene since trans is more stable. In cis alkene, two methyl groups are close to each other hence their electronic clouds repel each other.
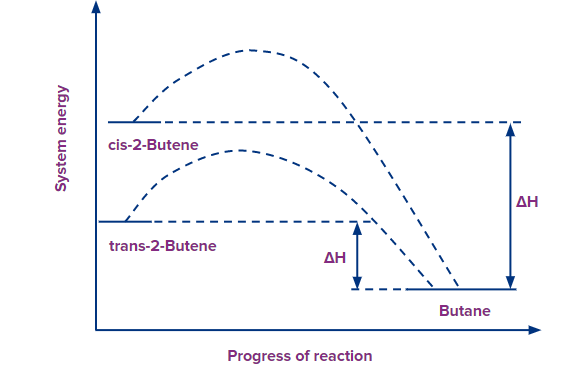
Similarly, as the hyperconjugation in an alkene increase, its stability increases. Hence, the heat of hydrogenation decreases.
Effect of Hyperconjugation on Bond Length
Bond length is affected by hyperconjugation. As the number of α-hydrogens increases, the extent of hyperconjugation also increases. Hence, the C = C (double bonds) gain more single-bond-like character and C−C (single bonds) gains more double-bond-like character, due to which the bond length of C=C increases and that of C−C decreases.
Example:
Arrange the bond lengths (x, y, z bonds) for the given molecules (ethene, propene, but-2-ene) in the decreasing order.
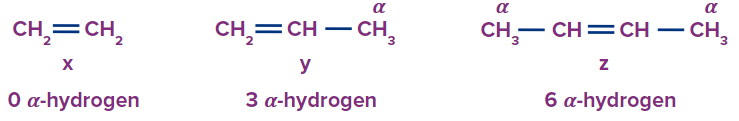
Ethene has no α-hydrogen, propene has three α-hydrogens, and but-2-ene has six α-hydrogens. Due to hyperconjugation, the double bond will have a partial double bond character. Hence, its bond length will be greater. So, the order of bond length will be: z > y > x
Dipole Moment
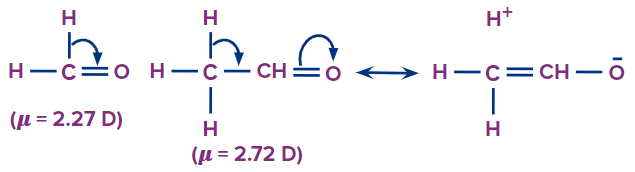
Since hyperconjugation causes the formation of charges, it also affects the dipole moment in the molecule. The increase in dipole moment, when the hydrogen of formaldehyde ( = 2:27 D) is replaced by a methyl group, i.e., acetaldehyde ( = 2.72 D) is due to hyperconjugation, which leads to the development of charges.
Electromeric Effect
When a reagent attacks a compound with multiple bonds, a full shift of pi electrons to one of the two atoms in the bond results. This is known as the electromeric effect. Polarity is created by this complete transfer of the shared pair of electrons.
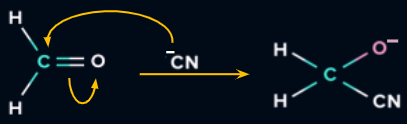
- This effect is frequently seen in organic molecules, whose structures typically include at least one double or triple bond.
- As mentioned, it is a temporary effect, so once the reagent is removed, the polarised molecule goes back to its unpolarised state (original state).
- Both electrophiles and nucleophiles have the potential to produce the electromeric effect.
- Another name for the electromeric effect is the E-effect.
Recommended Videos
Electron Displacement Effect - GOC Class 11 Chemistry One Shot Part 1 | NEET 2022-
Practice Problems
1. Compare the heat of hydrogenations of the following molecules.
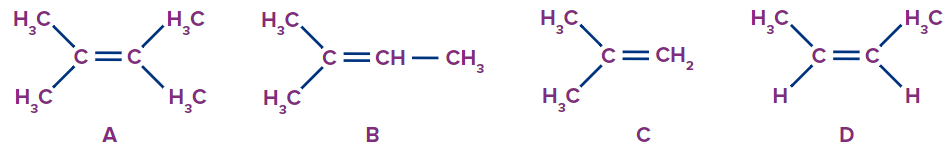
a. A > B > C > D
b. B > C > A > D
c. D > C > B > A
d. D > A > B > C
Answer: C
Solution: The heat of hydrogenation is inversely proportional to the stability of alkenes (or the number of α-hydrogens). Since ‘A’ has the highest number of α-hydrogens followed by ‘B’, the heat of hydrogenation will be less for ‘A’ than for ‘B’.
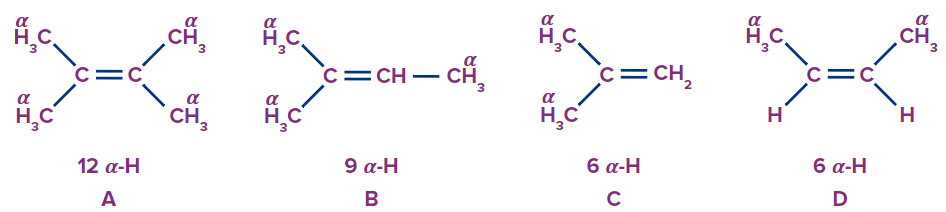
Comparing ‘C’ and ‘D’. ‘C’ (isobutene) is more stable than ‘D’. This can be explained using the hyperconjugation structures of ‘D’, in which the negative charge on carbon is destabilised by the methyl group attached to it due to its +I effect. Therefore, ‘D’ will have more heat of hydrogenation.
Thus, the heat of hydrogentation follows the order D > C > B > A..
So, option C is the correct answer.
2. Arrange the acidic order in the below-mentioned compounds.
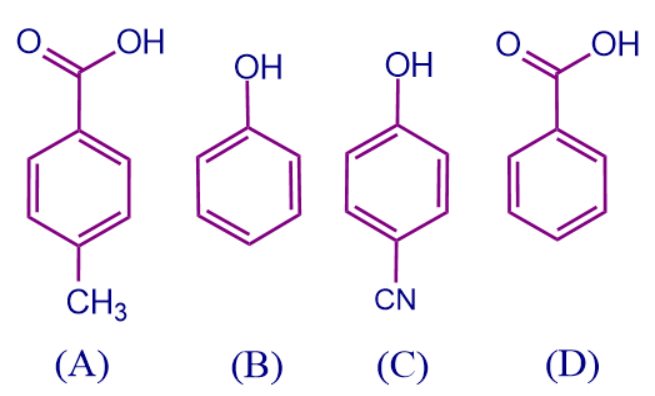
a. A > B > C > D
b. D > A > C > B
c. A > D > C > B
d. C > B > D > A
Answer: B
Solution: We can decide the order by checking the attached substituent groups and the electronic effects they are showing.
- In the above-mentioned compounds, compounds ‘A’ and ‘D’ are derivatives of benzoic acid and benzoic acid respectively, so they are more acidic than compounds ‘B’ and ‘C’ as carboxylic acids are more acidic than phenols.
- Among ‘A’ and ‘D’, ‘D’ is benzoic acid which has no substituent attached to it. In the compound ‘A’ -CH3 is attached to the benzoic acid ring, which shows +I effect and increases the electron density over the ring and thus removal of a proton is difficult. Thus, the order of acidic strength is D > A.
- Compounds ‘B’ and ‘C’ are phenol and phenol derivatives respectively, where -CN group attached in compound ‘C’ that shows -M effect. There is no substituents attached to the compound ‘B’. So on comparing, the -M effect increases the acidic strength of compound ‘C’ when compared to compound ‘B’. Thus, the order of acidic strength is C > B.
Therefore, the correct order of acidic strengths is D > A > C > B.
So, option B is the correct answer.
3. Which of the following shows −M effect?
a. -CHO
b. -CONH2
c. -COOCH3
d. All of these
Answer: D
Solution: When atoms or substituents that are directly attached to the conjugated system have −X=Y, where the electronegativity of Y is greater than that of X, they show the −M effect. Here, -CHO, -COOCH3, and -CONH2 have a −C=O group and the electronegativity of O is greater than that of C. Hence, all of these groups show the −M effect.
So, option D is the correct answer.
4. Which acid serves as the esterification's catalyst when carboxylic acid and alcohol are combined?
a. HNO3
b. Conc. H2SO4
c. H2SO3
d. All of these
Answer: B
Solution: Esters can be produced by the reaction of an alcohol and a carboxylic acid in an acidic medium, by removal of a water molecule. The reaction undergoes dehydration and we know that concentrated H2SO4 is a strong dehydrating agent.
So, option B is the correct answer.
Frequently Asked Questions – FAQ
1. Why do we say general organic chemistry is the backbone of organic chemistry?
Answer: The fundamental ideas in the broad topic of organic chemistry are covered in General Organic Chemistry (GOC). When studying subjects that are quite complex, having a solid grasp of the GOC ideas is essential (such as the mechanisms of named reactions). Hence, GOC is considered the backbone of organic chemistry.
2. What does "structure" in chemistry mean?
Answer: The arrangement of atoms within molecules is referred to as chemical structure. Butlerov came to understand that chemical compounds are structures with specific order rather than randomly arranged clusters of atoms and functional groups.
3. Is inductive effect distance dependent?
Answer: Yes, the inductive effect depends on the distance, as the distance increases, the inductive effect decrease due to a decrease in polarization. Generally, it is effective up to the third carbon. Its effect becomes negligible and insignificant after the third carbon bond.
4. Which has a bigger impact, inductive or mesomeric effect?
Answer: The mesomeric effect is thought to be more powerful and dominant than the inductive effect in the majority of substituents. Halogens are an apparent exception.


