-
Call Now
1800-102-2727
Galvanic Cells: Definition of Galvanic Cells, Function of Galvanic Cell, Salt Bridge, Practice problems and FAQs:
Place a zinc rod into copper sulphate solution in a beaker. What do you observe? The blue colur slowly decreases to give a colourless solution. And more importantly, the solution is found to be warmer as the reaction proceeds, according to the equation
as the reaction proceeds, the solution is found to be a little hotter also. The reaction taking place is:
Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
Similar results are observed when a rod of copper is placed in silver nitrate solution. The reaction taking
place is as follows:
When copper comes into contact with an aqueous silver salt solution, a similar results were observed. Copper displaces silver from aqueous silver nitrate to form copper sulphate solution with evolution of heat
Cu (s) + 2AgNO3(aq) → Cu(NO3)₂ (aq) + 2 Ag (S)
So, the redox reactions are exothermic, releasing some chemical energy, which gets dissipated into the atmosphere. We conclude that whenever a redox reaction takes place directly in a single beaker, chemical energy in the form of heat is produced. By suitable means, it is possible to bring about the redox reaction indirectly and utilize this wasted heat energy into an useful electrical energy. The set up used for this conversion is called a galvanic cell.
Come, let us know about this conversion of the chemical energy into an electrical energy.
Table of content
- Definition of Galvanic Cell
- Characteristics of Electrodes
- Salt bridge and its function.
- Criteria for selecting salt bridge
- Representation of Galvanic cell
- Practice problems
- Frequently Asked Questions (FAQs)
Definition of Galvanic Cell
A device used to convert chemical energy produced in a redox reaction into electrical energy is called a galvanic cell or voltaic cell, after the names of Luigi Galvani (1780) and Alessandro Volta (1800) who were the first to perform experiments on the conversion of chemical energy into electrical energy. Luigi Galvani and Alessandro Volta who observed first this phenomenon..
How a redox reaction can be used to produce electrical energy, leading to the formation of a galvanic cell, can be understood by taking the above two examples of redox reactions again.
Example: Redox reaction between Zn and CuSO4:
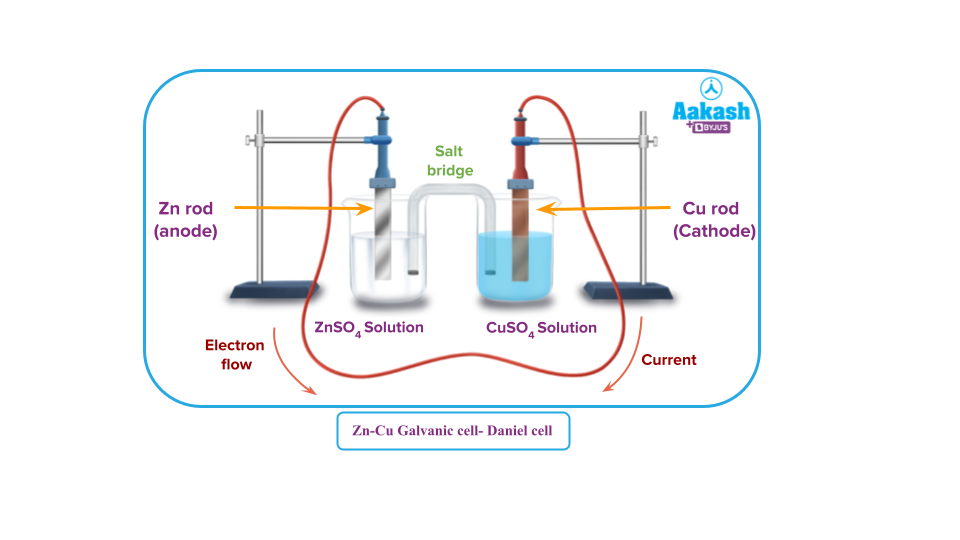
The reaction is
Zn + CuSO4 → ZnSO4 +Cu
Zn + Cu2+ → Zn2+ + Cu
reaction may be split into two half reactions as under:
ZnZn2++ 2 e-
Cu2+ + 2 e- Cu
The first reaction is called the oxidation half reaction and the second is called the reduction half reaction. The reaction obtained by adding the two half reactions is called the overall reaction.
Thus, if a redox reaction is allowed to take place in such a way that oxidation half reaction takes place in one beaker and the reduction half reaction in another beaker, the electrons given out by the former will be
taken by latter and a current will flow. The cell is thus made up of two half reactions or two half cells
Making of a Zn-Cu cell:
A zinc rod is placed in zinc sulphate solution taken in a beaker. A copper rod is placed in copper sulphate solution taken in another beaker. The two rods are connected by a wire and the two solutions are connected by a salt bridge.
Two beakers containing Zinc and Copper rods dipped inside their respective salt solutions separately are taken. The two rods are connected with an electrical wire. The solutions in the beakers are connected by what is called a salt bridge wetted with potassium nitrate solution to complete the electrical circuit.
2. Redox reaction between copper and silver nitrate
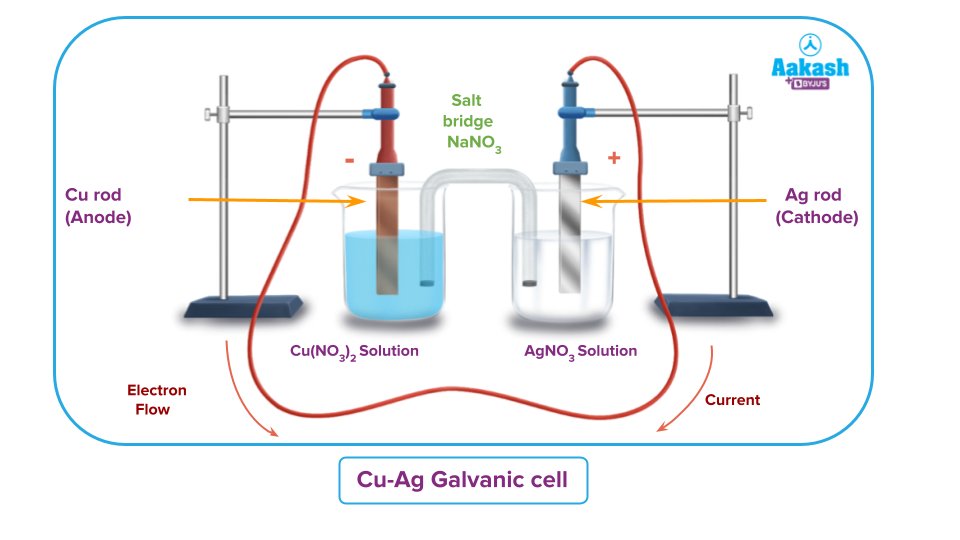
The reaction is:
Cu + 2AgNO3 → Cu (NO3)2 + 2 Ag
or it may be written as :
Cu + 2 Ag+ → Cu2+ + 2Ag
The two half reactions will be:
Cu → Cu2+ + 2 e- (Oxidation half reaction)
2 Ag+ +2 e- 2Ag (Reduction half reaction)
The copper electrode, T which the oxidation takes place is the anode.. Reduction takes place at the silver electrode, therefore, it acts as cathode. The conventional flow of current is from silver to copper.
In a similar manner, based upon any redox reaction, a galvanic cell can be constructed.
Characteristics of Electrodes
By convention, oxidation half cell is kept on the right with reduction half cell at the right
Anode
- Oxidation takes place at the anode.
- It acts as a source of electrons.
- It has negative polarity. (Not always true)
Cathode
- Reduction takes place at the cathode.
- It acts as a sink for electrons.
- It has positive polarity. (Not always true)
Salt bridge and its function.
A salt bridge is an inverted U-shaped tube containing concentrated solution of an inert electrolyte like KCI, KNO3, K2SO4, etc. or solidified solution of such an electrolyte in agar-agar and gelatine.
Salt bridge contains concentrated solution of inert electrolytes like KNO3, K2SO4, KCI or solidified electrolytes like gelatine and agar-agar placed in an inverted U tube. The salt used in the salt bridge has ions of almost similar mobility across electrodes..

Need for salt bridge
● Oxidation takes place on the anode and reduction takes place on the cathode.
● Zn is oxidized to Zn2+ ions which dissolve in the solution. Initially, this increases the concentration of Zn2+ ions in the solution while the sulphate ion concentration remains the same. The imbalance in the
charge (known as zeta potential) pushes back some of the Zn2+ ions as Zn. Hence, an equilibrium is established between Zn and Zn2+ ions stopping any further formation of ions and electrons.
Zn(s) ⇌ Zn2+(aq) + 2e-
For the working of the cell, this equilibrium needs to shift in the forward direction but as the concentration of Zn2+ ions increases the equilibrium will shift in the backward direction.
Now to shift this equilibrium, we have to neutralize the positive charge by adding negative ions. Copper ions in their solution accept electrons coming from the Zn oxidation to form copper atoms that get deposited on the cathode. This reduces the concentration of copper cations in the solution while retaining the sulphate anion concentration. Similar to the Zn solution, increasing the negative charge in the solution stops the reduction of copper ions to an equilibrium.
- The establishment of these two equilibria stops the electron flow through the circuit, and the cell comes to a standstill. Hence, to maintain the electron flow, there is a need for a salt bridge which can complete the circuit by providing both the positive and negative ions.
- In the anode half-cell, there is an excess of positive charge, thus, it pulls negative ions from the salt bridge to maintain electroneutrality and in the cathode half-cell there is an excess of negative charge, therefore, it pulls positive ions from the salt bridge to maintain electroneutrality.
Role of salt bridge
- If electrodes are not connected internally, the cell generates electrical energy initially but after some time the electrodes get polarized, i.e., accumulation of oppositely charged ions around the electrodes takes place. and the cell stops working or the flow of electrons stops.
- For the electrical circuit to be closed the two electrolytes also are connected.by the salt bridge.
- From the salt bridge electrolyte (say KCl): Cations (K+) migrate towards the cathode and anions (Cl-) migrate towards the anode and polarize some of the oppositely charged ions in the solution
towards themselves. Thus, the cell keeps working and does not stop.
- If there is no salt bridge then there is no flow of electricity after some time because there will be an excess positive charge in the anode half-cell (left beaker) and an excess negative charge in the cathode half-cell (right beaker).
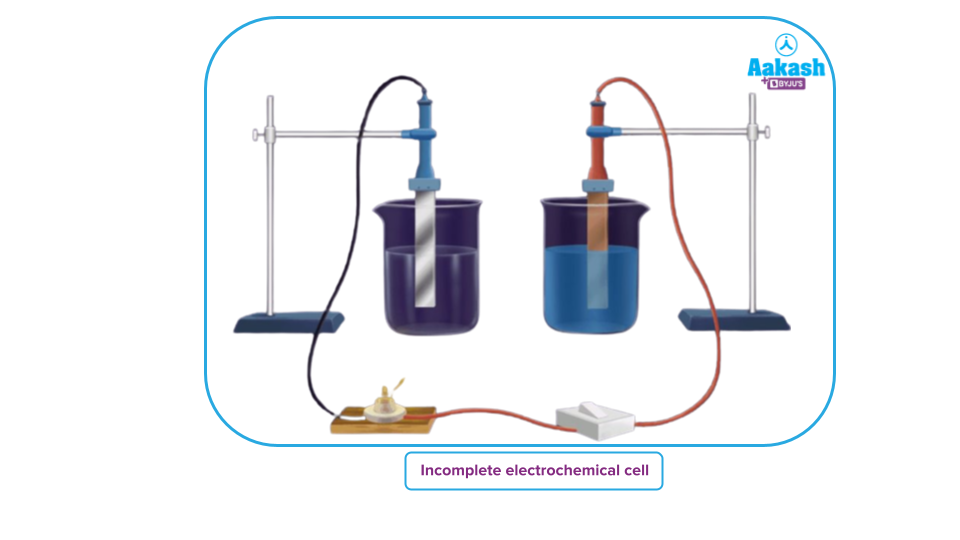
After the introduction of a salt bridge, electroneutrality is maintained as the counter ions are provided by the salt bridge and the flow of charge takes place (the bulb will light up again).
Criteria for selecting salt bridge
- Should consist of cations and anions having same mobility.
- Should not react with the ions in the solution.
- Should not get oxidized/reduced at the electrodes.
Representation of Galvanic cell
Galvanic cell is represented in a manner as illustrated below for the Daniell cell.
Zn | Zn2+ (c₁) || Cu2+ (c2) | Cu
- By convention, the electrode on which oxidation takes place(oxidation half cell) is written on the left hand side and the other electrode on which reduction takes place is written on the right hand side.
- The Oxidation half cell on the left hand side is written in order starting with the symbol of the metal (or the gas) first, followed by the symbol of the ion in equilibrium with its concentration in brackets. The Reduction half cell is written in th reverse order of the ion in equilibrium with concentration in bracket followed by the symbol of the cathode metal. (or the gas).
- Single vertical lines separate the metal electrode and the the ion in equilibrium for the both half cell.
- The two half cell is separated by a double vertical line and indirectly indicates the presence of a connecting salt bridge connecting the two electrolytes..
Metal | Metal ion (conc.) || Metal Ion (conc.) | Metal
Anode(Oxidation) Salt bridge Cathode (Reduction)
Concentration cell
- Concentration cells are electrochemical structures made up of two half-cells with identical electrodes but different concentrations. The more concentrated half cell is diluted and the less concentrated half cell has its concentration increased as the cell as a whole works to achieve equilibrium through the transfer of electrons between these two half cells. Thus, a potential difference is produced as the cell moves toward chemical equilibrium.
- Generally concentration cells has been classified into two categories
- Electrode concentration cell
- Electrolyte concentration cell
Practice problems
Q. Which of the following electrolytes is not preferred in a salt bridge?
a) KCl
b) KNO3
c) NH4NO3
d) NaCl
Among the following electrolytes, which one is suitable as a salt bridge?
(A) AlNO3
(B) CsCl
(C) NaNO3
(D) NH4NO3
Answer: (C)
Solution: In a salt bridge, the electrolytes like NaNO3 ,KCl, KNO3 or NH4NO3 only are preferred because their ions have almost equal transport number, viz., 0.5, i.e., they have the same mobile speed under the influence of the current. Correct answer is option C.
Q. A cell is prepared by dipping a copper rod in 1 M CuSO4 solution and an iron rod in 2 M FeSO4 solution. What are the cathode and anode respectively?
A cell is constructed using a copper rod placed in 0.5 M CuSO4 solution and iron rod placed in 1 M FeSO4 solution as two half cells. Identify, which of them will act as cathode and anode.
a) Cathode: Iron, Anode: Copper
b) Cathode: Copper, Anode: Iron
c) Cathode: Iron, Anode: Iron
d) Cathode: Copper, Anode: Copper
- Iron electrode: Cathode, Copper electrode: Anode
- Copper electrode: Cathode, Iron electrode: Anode
- Iron electrode: Cathode, Iron electrode: Anode
- Copper electrode: Cathode, Copper electrode: Anode
Answer: (B)
Solution: The given cell is represented as:
Fe (s) | FeSO4 (1 M) || CuSO4 (0.5 M) | Cu (s)
The standard reduction potential of iron is less than that of copper. So, the easily oxidizable iron will be anode with copper acting as a anode..
Q. Identify the right arrangement of activity of the metals.
a) Zn > Mg > Fe > Cu > Ag
b) Zn > Mg > Fe > Ag > Cu
c) Mg > Zn > Fe > Ag > Cu
d) Mg > Zn > Fe > Cu > Ag
a) Mg> Zn > Cu > Fe > Ag
b) Mg > Fe> Zn > Ag > Cu
c) Mg > Zn > Fe > Ag > Cu
d) Mg > Zn > Fe > Cu > Ag
Answer: (D)
Solution: Greater the oxidation potential of metal, the more easily it can lose electrons and hence greater is its reactivity. As a result, a metal with greater oxidation potential can displace metals with lower oxidation potentials from their salt solutions metals with lower oxidation potentials resist from getting oxidized than metals with higher oxidation potential. The order of activity is directly related to the magnitude of oxidation potential. So the order of oxidation potential and hence the activity of the metals are in the order Mg > Zn > Fe > Cu > Ag.
Q. Which of the following is false regarding galvanic cells?
a) It converts chemical energy into electrical energy
b) The electrolytes taken in the two beakers are different
c) The reactions taking place are non-spontaneous
d) To set up this cell, a salt bridge is used
Answer: (C)
Solution: Galvanic cells are used to convert chemical energy into electrical energy. Two electrodes are usually set up in two separate beakers. The electrolytes taken in the two beakers are different. Galvanic cells are based upon spontaneous redox reactions. A salt bridge is used to set up this cell.
Q. Select the wrong statement among the following with respect to a galvanic cell.
- The reactions which are taking place in the galvanic cell are non-spontaneous
- Galvanic cells converts chemical energy into electrical energy
- Salt bridge is used to set up galvanic cell
- In two different beakers different electrolytes are taken
Answer: (a)
Solution: In galvanic cells, spontaneous redox reactions occur. Galvanic cells converts chemical energy into electrical energy. To construct a galvani cell, salt bridge is must. The two different electrolytes are taken in two different beakers.
Frequently Asked Questions (FAQs)
Q.1 How a galvanic cell stops working?
Answer: The positive charge around anode prevents electrons to flow out from it so the potential difference becomes zero and the cell stops after sometime.
Q.2 Do galvanic cell require electricity?
Answer: Galvanic cells derives its energy from spontaneous redox reactions, while electrolytic cells involve non-spontaneous reactions and thus require an external electron source like a DC battery or an AC power source.
No. From spontaneous redox reactions, galvanic cell can generate the energy. So, it does not need any external electricity source for work.
Q.3 Can we run a galvanic cell forever?
Answer: Galvanic cells go “dead” for several reasons. One reason may be that the electrode which is the anode (being oxidized) may simply be used up, i.e. there are no more atoms to remove electrons from.
Galvanic cells may discharge for many reasons. Generally, the anode can get oxidized naturally and be consumed even without being used for generating electricity.So after some time there will not be enough anode to release electrons and complete the circuit.
Q.4 What kind of batteries we can construct from galvanic cell?
Answer: A battery is made up of a collection of galvanic cells that are wired together to create a single voltage source. A typical 12V lead-acid battery, for instance, has six galvanic cells connected in series, each with lead anodes and lead dioxide cathodes that are submerged in sulfuric acid.


