-
Call Now
1800-102-2727
Ethers Nomenclature: Introduction, Classification, Nomenclature of Ethers, Practice Problems & Frequently Asked Questions
Ethers are a class of organic compounds finding many applications. For example, are you aware of the chemical added to beverages as a flavouring agent? It’s Ether
Some of the newly developed ether compounds, such as diethyl ether, are very helpful in the production of perfumes or fragrant goods, such as soaps, detergents, and everyday household items like oils, medications, etc.

Drinks have a fruity-green, exotic flavour owing to 1-methoxy-3-hexanethiol, which gives a grapefruit aroma to beverages.
Some ethers are also used to add salty scents, notably meat-like aromas, to foods such as soup and broths, spices, snacks, sauces, or ready-to-eat non-vegetarian meals in order to give them the traditional flavour of the meat.
Anise seed essential oil frequently contains methyl benzene (anisole) as a fragrance-enhancing component.
Ethers are also used as solvents in laboratories and industries.
Come, let us know about the classification and, naming of ethers
Table of Contents:
- Introduction of Ethers
- Classification of Ethers
- Ethers Nomenclature
- Practice Problems
- Frequently Asked Questions(FAQs)
Introduction of Ethers:
Ethers are chemical compounds with two identical or dissimilar alkyl or aryl groups bound to an oxygen atom. The general formula for ethers can be written as R-O-R, R-O-Ar, or Ar-O-Ar. Ar stands for an aryl group, while R stands for an alkyl group.
Tetrahedral geometry is present in ethers, where sp3 hybridization of oxygen exists. The C-O bonds are somewhat polar because oxygen has a higher electronegativity than carbon. Ethers acquire a net dipole moment owing to this.
Classification of Ethers:
Based on the nature of the groups that are joined to oxygen, the classification of ethers is done. Thus, we can divide them into the following categories:
1. Simple ethers or Symmetrical ethers:
On both sides of the functional group -O-, these ethers are joined by identical alkyl or aryl groups.
Example: CH3-O-CH3 (dimethyl ether), C2H5-O-C2H5 (diethyl ether).
2. Mixed ethers or unsymmetrical ethers:
Along either end of the functional group -O-, these ethers are joined by various alkyl or aryl groups.
Example: C3H7-O-CH3 (methyl propyl ether), CH3-O-C6H5 (methyl phenyl ether).
3. Cyclic ethers:
Heterocyclic substances called cyclic ethers have an atom of oxygen in a saturated ring. Many cyclic ethers have well-known names and are often employed as solvents because of their inert nature. Cyclic ethers include epoxide.
Epoxide is the name for the cyclic ether having a three-atom ring. The fundamental building block of an epoxide is an oxygen atom connected cyclically to two nearby hydrocarbon carbon atoms. Now, this ring is stretched and therefore very reactive in comparison to other ethers because it approximately resembles an equilateral triangle.
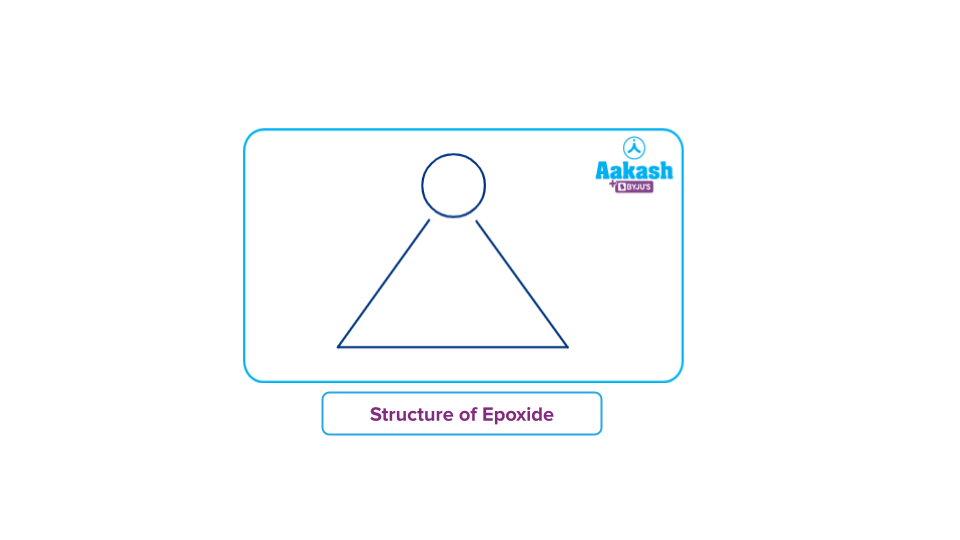
4. Crown ethers:
These are cyclic polyethers where two or three carbon atoms are posited between each of the two, four, or more oxygen atoms forming a ring. Crown ethers are known to interact with metal ions to produce more stable complexes than simple ethers. The attraction between the positively charged ions and the oxygen atoms' nonbonding electrons causes the binding to take place. Crown ethers enable the dissolution of inorganic salts in nonpolar organic solvents, enabling the conduct of several processes in nonpolar solvents.
Crown ethers work by locking the cations within a cavity that is hydrophilic while the outer shell, which is made up of C-H bonds, is hydrophobic, making them useful for dissolving ionic compounds in organic solvents, such as KMnO4 in toluene.
Ethers Nomenclature:
A) Common Names of linear Ethers:
The general or common nomenclature of ethers adheres to the principle of designating various alkyl/aryl groups that are connected to the oxygen atom along either side in alphabetical sequence, followed by the word ether.
For example, the name C2H5 O CH3 is given by Ethyl methyl Ether.
Greek number prefixes like "di" are used to denote the oxygen atoms of ethers that are connected to the identical group along either side. The suffix "di" is placed before the aryl/alkyl groups that are linked to the oxygen atom to give various types of ethers their names.
For example, the name C2H5 O C2H5 is known by Diethyl Ether.
B) IUPAC Nomenclature of Ethers:
IUPAC ether nomenclature is subjected to a number of rules. According to IUPAC terminology, ethers are considered substituted hydrocarbons.
Of the hydrocarbons attached to the oxygen atom, the hydrocarbon with the longest carbon chain is considered the parent hydrocarbon. The other hydrocarbon along with the oxygen is considered than alkoxy substituent.
For example, C2H5- O C2H5 is named in IUPAC as Ethoxy Ethane.
Let's look at some ethers in tabular form with their common names and IUPAC nomenclature.
|
Formula |
Common Name |
IUPAC Name |
|---|---|---|
|
C2H5 O C2H5 |
Diethyl Ether |
Ethoxy Ethane |
|
CH3 O CH3 |
Dimethyl Ether |
Methoxy Methane |
|
C2H5 O CH3 |
Ethyl Methyl Ether |
Methoxy Ethane |
|
CH3 O CH(CH3)2 |
Methyl isopropyl Ether |
2-Methoxy Propane |
|
C6H5 O C6H5 |
Diphenyl Ether |
Phenoxy benzene |
|
C6H5 O CH3 |
Anisole or Methyl Phenyl Ether |
Methoxy benzene |
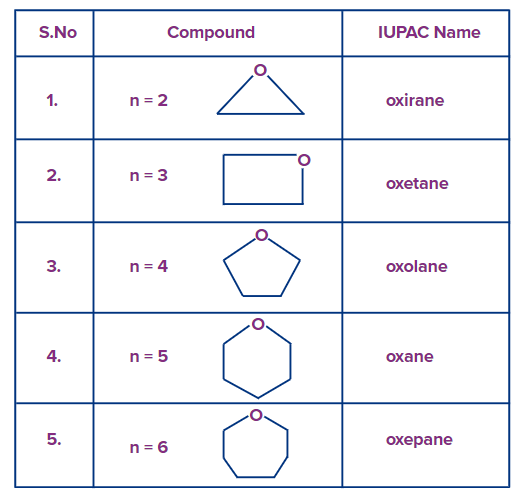
C) Common Names of Cyclic Ethers:
To denote the number of carbon atoms in the three-, four-, five-, six-, and seven-membered ring systems, respectively, the IUPAC nomenclature for cyclic ethers uses the suffixes "irane," "etane," "olane," "ane," and "epane" following the prefix "ox."
Below are the compounds' IUPAC names and structures.
D) IUPAC nomenclature of crown ethers:
A ring having 18 membered rings and six oxygen atoms is referred to as 18-crown-6. The structure of this crown ether is given below;
Practice Problems:
Q1. The dimethyl ether C-O-C bond angle is:
(A) 110o
(B) 120o
(C) 180o
(D) 100o
Answer: (A)
Solution: Dimethyl ether has a bent structure. We are aware that the oxygen atom has a higher electronegativity than the carbon atom. Both the methyl group -CH3 and the oxygen atom are joined. Two lone pairs exist on the oxygen atom, and they will work to remain close to it. This molecule has a C-O-C bond angle of 110o as a result of the two methyl groups that are present and the lone pairs of electrons that repel one another.
Q2. Ethers have a boiling point that is _____ than alcohols with a similar molar mass.
(A) lower
(B) greater
(C) Similar to
(D) slightly lower
Answer: (A)
Solution: Because there is a lot of intermolecular hydrogen bonding, alcohol has a greater boiling point than ether. The ether is substantially less dense than the comparable alcohol because there are no hydrogen bonds to organise the liquid's structure.
Q3. Ethers can be employed as solvents since they react only with the below reactants.
(A) Reducing agents
(B) Acids
(C) Oxidising agents
(D) Bases
Answer: (B)
Solution: Ethers are resistant to bases and nucleophile attacks. Ethers are typically less reactive than cations, but they are excellent solvents in many organic reactions because they can solvate cations by donating the electron pair from oxygen atoms. Ethers only react with acids because hydrogen ions can attack the lone pair of oxygen.
Q4. What among the following is the right justification for the miscibility of cyclic ether with water?
(A) Hydrogen Bonding
(B) Planar structure
(C) Molar Mass
(D) None of the above
Answer: (A)
Solution: In contrast to linear aliphatic ethers, cyclic ethers like 1,4-dioxane and tetrahydrofuran have more available oxygen atoms for hydrogen bonding. As a result, they are soluble(miscible) in water.
Frequently Asked Questions(FAQs):
Q1. Why are ethers explosive when exposed to air?
Answer: When exposed to air or ozonized oxygen in the presence of sunshine or UV light, ethers create a peroxide bond with oxygen. These peroxides have a very toxic character. They are greasy liquids that violently disintegrate even at low concentrations. As a result, we must take care to never dry out esters. It might trigger violent reactions.
Q2.What made ether ineffective as an anaesthetic?
Answer: As safer, more efficient inhalation anaesthetics were developed, the utilisation of chloroform and ether gradually decreased until they were no longer employed in surgical procedures. The 20th century saw attacks on chloroform in particular after research mice and rats were used to demonstrate that it was carcinogenic when consumed.
It is presently mostly utilised in the production of fluorocarbons, which are refrigerants and aerosol propellants. It is also used in some cough and cold medications, dental treatments (such as toothpaste and mouthwash), topical liniments, and other goods.
Q3. Why does ethoxy ethane have identical solubility as butan-1-ol?
Answer: The solubility of ethoxyethane and butanol in water with the same molar mass is approximately the same, with 7.5 g for ethoxyethane and 9 g for butanol per 100 mL of water. This is due to the oxygen in ethers forming a hydrogen -bond with the water molecule, much like it does in alcohols.
Q4. At ambient temperature, why is dimethyl ether a gas and ethanol (ethyl alcohol) a liquid?
Answer: Because hydrogen bonds between molecules keep them closely bound to one another, ethanol (ethyl alcohol) is a liquid at ambient temperature. The molecules remain strongly bound together because of the hydrogen bonding between the oxygen atoms of one molecule and the hydrogen atom of a nearby ethanol (ethyl alcohol) molecule.
In contrast, dimethyl ether has weak van der-Waal forces and no hydrogen bonds, which causes it to be a gas at ambient temperature.


