-
Call Now
1800-102-2727
Synthesis and Reactions of Epoxides: Structure, Synthesis and Reactions of Epoxides, Practice problems and Frequently Asked Questions(FAQs)
Epoxides feature a three-membered ring and in the center one oxygen atom. Despite the lack of a sufficient leaving group, they are associated with significant ring tension, which is the basis of their reactivity towards nucleophiles.
Despite the fact that oxygen is a weak leaving group, the epoxide's ring strain aids in the reaction's completion. Because the angle between the carbons is 60o instead of 109.5o, as it should be for sp3 hybridized tetrahedral atoms, the ring strain is caused by the geometry of the carbons not being appropriate.
Because the ring strain is not as significant, larger cyclic ethers would not be susceptible to acid- or base-catalyzed cleavage under the same conditions as the three-membered epoxide ring.
Table of content:
- Structure of epoxides
- Synthesis of epoxides
- Reactions of epoxides
- Practice problems
- Frequently asked questions(FAQs)
Structure of epoxides:
One epoxide's basic structure consists of an oxygen atom linked to two neighboring hydrocarbon carbon atoms in a cyclic manner. The general ethers, on the other hand, can be thought of as a set of chemical molecules that contain an ether group. This group is made up of two alkyl or aryl groups linked by an atom of oxygen. In general, the formula is ROR', where R and R' denote the alkyl or aryl groups respectively. As a result, it may be determined that its fundamental structure has two hydrocarbon carbon atoms connected to an oxygen atom.
The cyclic ether with a three-atom ring is known as an epoxide. Now, because this ring approximates an equilateral triangle, it is strained and thus extremely reactive in comparison to other ethers. The oxidation of ethylene over a silver catalyst produces it.
Synthesis of epoxides:
1. When ethylene reacts with oxygen in the presence of a silver catalyst, epoxide is formed. It can be represented chemically as:
7 CH2 = CH2 + 6 O2 + Ag → 6 C2H4O + 2 CO2 + 2 H2O
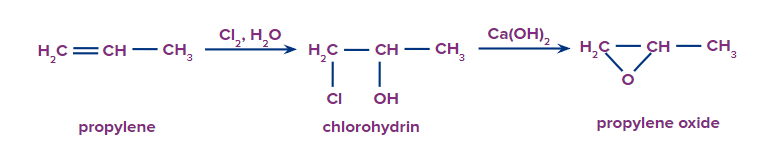
2. Another important industrial route to epoxides requires a two-step process. An alkene is converted to a chlorohydrin, which is then treated with a base to remove the chloride as a leaving group and yield the epoxide; this is the process by which propylene oxide is produced.
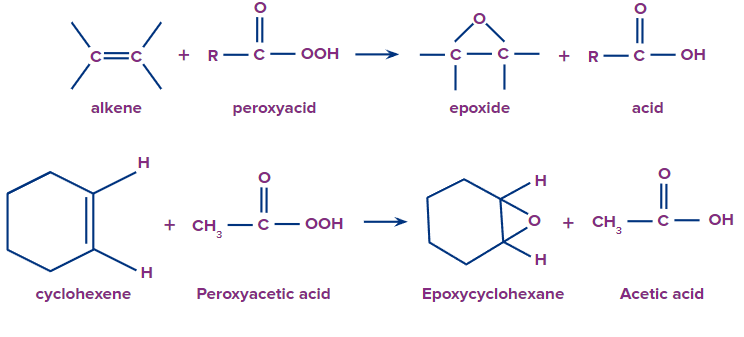
3. The epoxidation of alkenes results in a more complex type of epoxide. In this procedure, peroxy acid (RCO3H) is used to transfer one oxygen atom.
Reactions of epoxides:
1. Epoxide ring opening by hydrolysis:
Trans-1,2-diols can be produced via hydrolysis of epoxides (1,2 diols are also called vicinal diols or vicinal glycols). The reaction can take place in either acidic or basic conditions, resulting in regioselectivity.
Acid catalyzed hydrolysis:
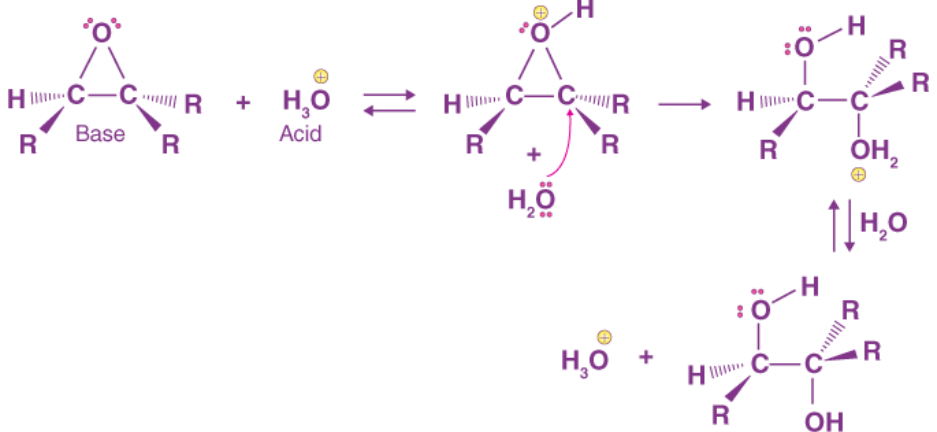
In aqueous acidic conditions, the epoxide oxygen is protonated and then attacked by a nucleophilic water. A 1,2-diol product is formed after deprotonation to reform the acid catalyst. If the epoxide is asymmetric, the incoming water nucleophile will attack the epoxide's more substituted carbon first. An SN1 like process opens the epoxide ring in acidic medium, causing the product's two -OH groups to be trans to each other.
Base catalyzed hydrolysis:
The attack of a hydroxide nucleophile opens the epoxide during an SN2 reaction in aqueous basic conditions. The oxygen epoxide reacts with aq NaOH to form an alkoxide, which is then protonated to produce the 1,2-diol product. If the epoxide is asymmetric, the incoming hydroxide nucleophile will attack the less substituted epoxide carbon first. The two -OH groups in the product will be trans to each other since the reaction is catalyzed by an SN2 mechanism.
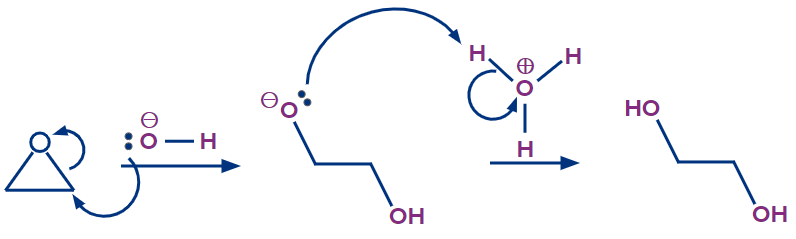
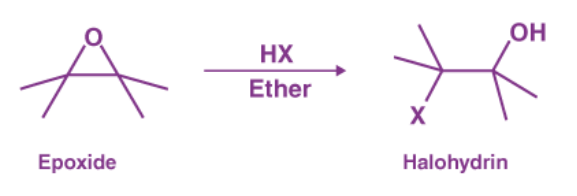
2. Epoxide ring opening by HX:
Trans halohydrins can be formed by opening epoxides with anhydrous acids (HX). The halogen anion will attack the less substituted carbon via an SN2 reaction when both epoxide carbons are primary or secondary. If one of the epoxide carbons is tertiary, the halogen anion will attack it primarily in an SN1 manner.
3. Epoxide ring opening by nucleophiles:
To open the ring of an epoxide, a variety of basic nucleophiles such as amines, Grignard reagents, acetylide anions, and hydride can be used. An SN2 mechanism is usually responsible for these ring openings.
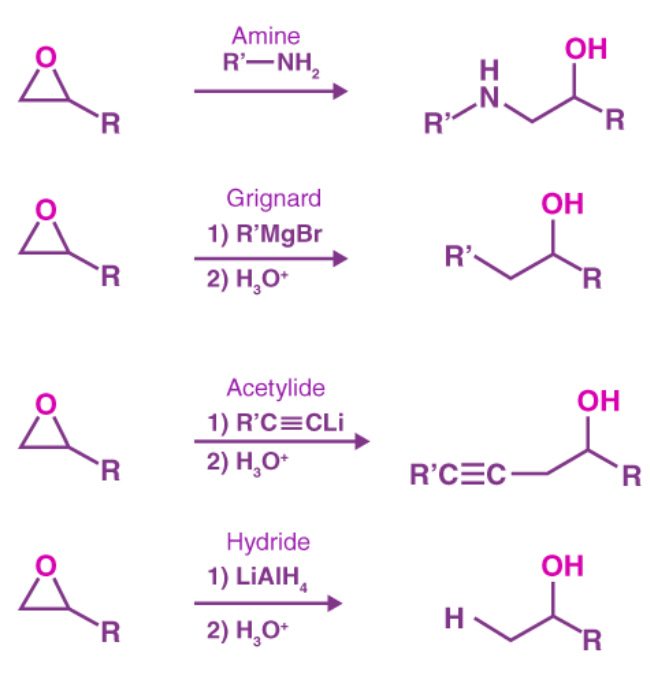
Grignard reagent reactions with ethylene oxide are particularly useful because they produce a primary alcohol with two extra carbon atoms than the original Grignard reagent.
Because Grignard reagents are both strong nucleophiles and strong bases, this reaction follows the same SN2 mechanism as the basic epoxide ring opening. In the first step of the Grignard reaction's mechanism, the SN2 attack opens the epoxide to form an alkoxide. In the second step of the mechanism, the alkoxide is protonated to form alcohol.

4. Epoxide ring opening by alcoholysis:
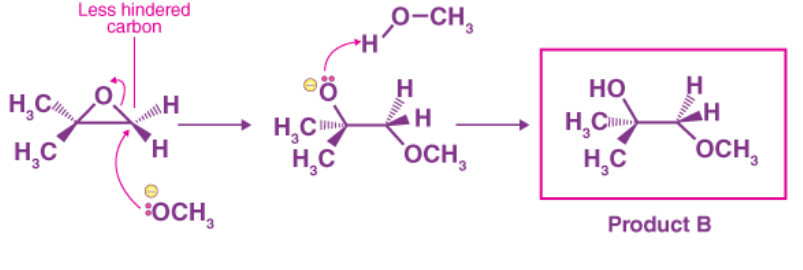
Ring-opening reactions can occur via SN2 or SN1 processes, depending on the type of epoxide and the reaction conditions. If the epoxide is asymmetric, the resulting structure will vary depending on which mechanism dominates. When an asymmetric epoxide undergoes alcoholysis in basic methanol, an SN2 reaction opens the rings, and the less substituted carbon becomes the target of nucleophilic attack, yielding product B:
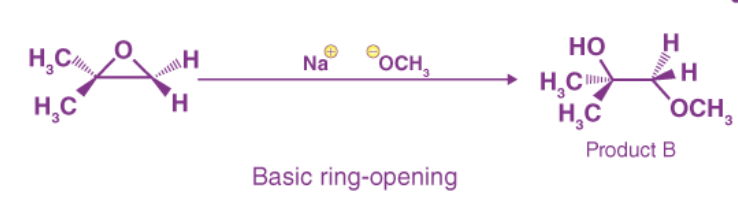
However, when solvolysis occurs in acidic methanol, the reaction follows an SN1-like mechanism, with the more substituted carbon being attacked. As a result, product A is dominant:
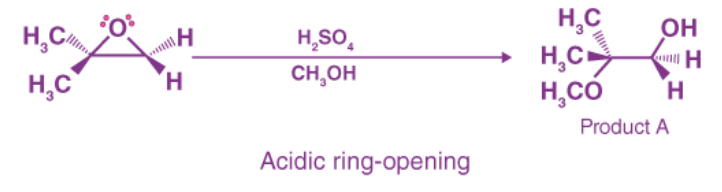
Basic Epoxide Ring-Opening:
The leaving group in the basic SN2 reaction is an alkoxide anion since there is no acid available to protonate the oxygen prior to ring opening. Because alkoxide is a poor leaving group, the ring is unlikely to open without the help of the nucleophile.
A deprotonated, negatively charged methoxide ion is extremely powerful as a nucleophile. The SN2 mechanism is very likely to be used when a nucleophilic substitution process involves a weak leaving group and a strong nucleophile.
Despite the presence of two electrophilic carbons in the epoxide, the least hindered carbon is the best target for the nucleophile in an SN2 reaction. This explains the observed regiochemical result . The source of nucleophilic attack is the same as in other SN2 reactions.
Acid-Catalyzed Epoxide Ring-Opening:
The acid-catalyzed epoxide ring-opening reaction is best defined as a hybrid or cross of the SN2 and SN1 mechanisms. The oxygen is protonated first, yielding a suitable leaving group (step 1 below). Step 2 shows the carbon-oxygen bond begin to break, allowing positive charge to accumulate on the more substituted carbon.
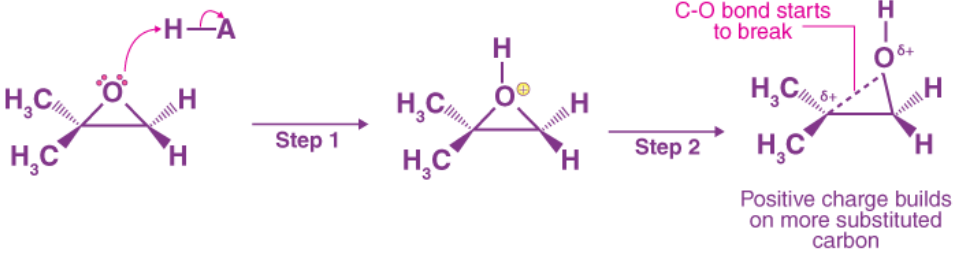
Unlike in an SN1 reaction, the nucleophile attacks the electrophilic carbon (step 3) before a full carbocation intermediate is developed.
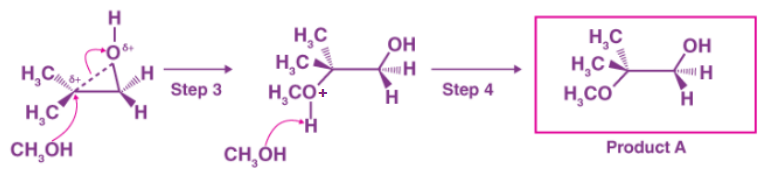
Because the carbon-oxygen bond is still intact to some extent and the oxygen prevents attack from the front side, attack occurs preferentially from the backside (as in an SN2 reaction). However, the regiochemical outcome differs from the base-catalyzed reaction: in the acid-catalyzed reaction, the nucleophile attacks the more substituted carbon because it has a higher degree of positive charge.
5. Additional stereochemical considerations of ring opening:
Because of the regiochemical control of the reaction, one stereoisomer can be produced in excess during the ring-opening of an asymmetrical epoxide. If the epoxide is symmetrical, then each carbon has nearly the same ability to accept the incoming nucleophile. When this happens, the product usually has a mixture of enantiomers.
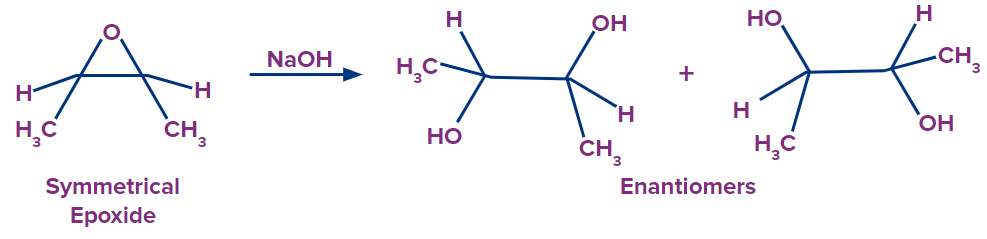
Practice problems:
Q.1. Which of the following Is the correct reason for cyclic ether to be miscible in water?
(A) Planer structure
(B) Larger size
(C) Molecular weight
(D) Hydrogen bonding
Answer: (D)
Solution: Tetrahydrofuran and 1,4-dioxane are cyclic ethers that are miscible with water. The polarity of these ether molecules is stronger than that of alkyl ethers (chain ethers) because the oxygen atoms are more exposed outside the molecule for hydrogen bonding.
Q.2. Why are epoxides polar?
(A) Due to high electronegativity difference between carbon and oxygen
(B) Due to low electronegativity difference between carbon and oxygen
(C) Due to ring strain
(D) None of the above
Answer: (A)
Solution: The epoxide unit of a three-membered ring has two C atoms and one O atom. The atoms are linked by Sigma bonds. Both C-O bonds are polar due to the high electronegativity difference between the C and O atoms.
Q.3. Cyclic ethers with six membered ring systems are known as
(A) Oxiranes
(B) Tetrahydropyran
(C) Oxetanes
(D) None of the above
Answer: (B)
Solution: Cyclic ethers are heterocyclic compounds that contain an oxygen atom as well as carbon atoms in the ring. They are named for the number of carbon atoms in their ring structure. Cyclic ethers with three membered ring systems are termed "oxirane," four membered ring systems are called "oxetane," five membered ring systems are named "Tetrahydrofuran" and six membered ring systems are named as “Tetrahydropyran”.
Q.4. Epoxides are
(A) Electrophiles
(B) Nucleophiles
(C) Both
(D) None of the above
Answer: (A)
Solution: Because substantial ring strain is relieved when the ring opens upon nucleophilic attack, the carbons in an epoxide group are highly reactive electrophiles. Epoxides are typically generated in the cell and laboratory by the oxidation of an alkene. Epoxides are electrophilic due to their strained three-membered ring configuration, where nucleophile attack and carbon release the ring strain.
Frequently asked questions:
Ques1. What are cyclic ethers?
Answer: Cyclic ethers are heterocyclic compounds that contain an oxygen atom as well as carbon atoms in the ring. They are named for the number of carbon atoms in their ring structure. Cyclic ethers with three membered ring systems are termed "oxirane," four membered ring systems are called "oxetane," five membered ring systems are named "Tetrahydrofuran". The cyclic nature of these rings imposes angle strain, which is greater in the ring with fewer carbon atoms. A three-membered ring system, for example, is more strained than ring systems with more carbon atoms.
Ques2. What are crown ethers?
Answers: Crown ethers are cyclic polyethers having four or more oxygen atoms separated by two or three carbon atoms in each molecule. Crown ethers have the generic formula (OCH2CH2)n or (OCH2CH2CH2)n, and are named after the total number of atoms in the ring as well as the number of oxygen atoms in the ring. As a result, 18-crown-6 is a ring with 18 members and six oxygen atoms. A hollow at the center of all crown ethers is lined with oxygen atoms and can hold an alkali metal ion like K+. The cation is stabilized by interacting with the surrounding oxygen atoms' lone pairs of electrons.
Ques3. What kind of solvent is used in epoxidation?
Answer: A nonaqueous solvent such as chloroform, ether, acetone, or dioxane is used in either case. This is because the epoxide ring is hydrolyzed in an aqueous media with any acid or basic catalyst present to form a vicinal diol, a molecule with two -OH groups on neighboring carbons.
Ques4. Do epoxides have a high reactivity?
Answer: An epoxide is a three-atom ring cyclic ether. Because this ring resembles an equilateral triangle, it is strained and thus more reactive than other ethers. They are produced in large quantities for a variety of applications.


