-
Call Now
1800-102-2727
Elimination Reactions: Introduction, Types, 𝝰-Elimination, 𝛃-Elimination, Cyclisation Elimination, Practice Problems, FAQs
Have you ever been to a football match?
If not then you must have seen it on the TV. There are 11 players in the football match in a team. There is a certain code of conduct that has to be maintained by each player on-field during the match. If any violation occurs by a player then, the referee reserves the right to eliminate the player by showing a red card. A player can also be eliminated if he gets some serious injury while playing on the field.

Similarly in organic reactions, a molecule gets eliminated from the compound under a variety of conditions. Such reactions are known as elimination reactions.
Let's study what all types of elimination reactions are possible!
TABLE OF CONTENTS
- Introduction
- Types
- 𝝰-elimination
- 𝛃-elimination
- Types of -elimination
- Practice Problems
- Frequently Asked question-FAQs
Introduction:
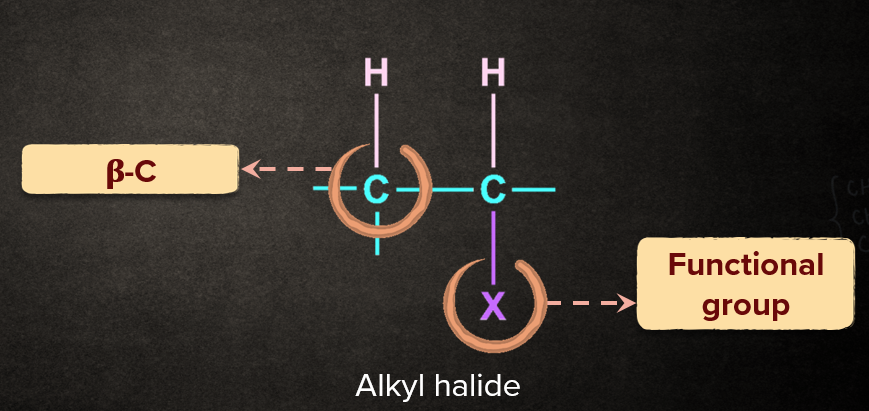
An organic reaction in which two or more groups/substituents/atoms are removed from a molecule.The general representation of elimination reaction as below.
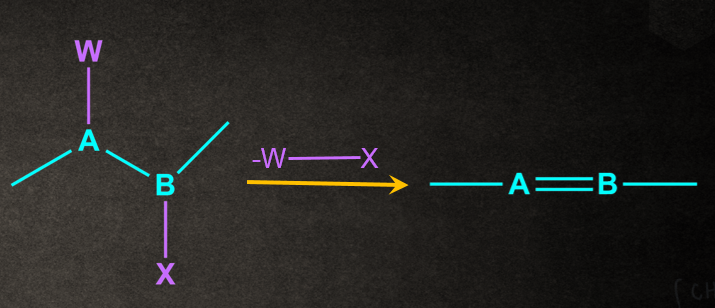
In general, elimination reactions can be considered as the reverse of addition reactions.
The below-given reaction is an example of an addition reaction, where a molecule of water adds up to alkene to form one molecule of ethanol.
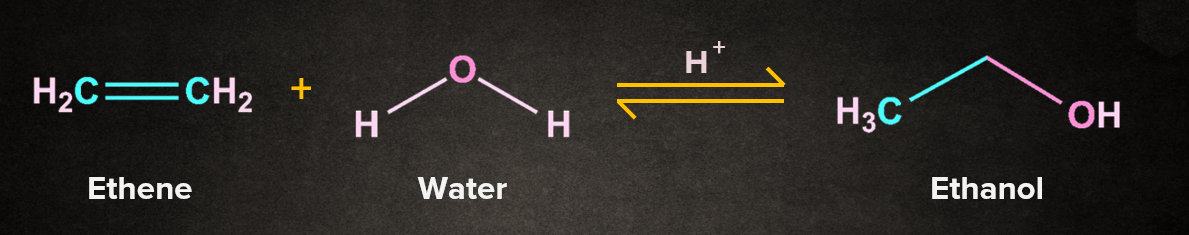
The below given reaction is an example of an elimination reaction, where a molecule of water gets eliminated to give one molecule of ethene.

The above two examples show that the elimination reaction is the reverse of addition reaction.
Types of elimination reaction:
Based on the fashion in which the atoms/groups removed from the same atom or different atoms in a molecule, elimination reactions are of two types.
1. 𝝰-elimination
2. 𝛃-elimination
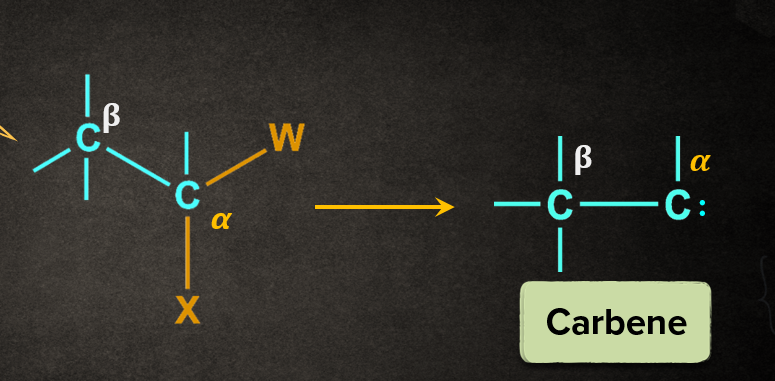
𝝰-elimination:
The removal of two atoms or groups attached to the same atom is known as 1,1-elimination or 𝛂-elimination. Both the eliminating substituents eliminate from the same carbon to give an intermediate, carbene.
The below given reaction is a general representation of 𝛂-elimination, where X and W gets eliminated from the 𝛂-position to form carbene.
Example- Reimer‒Tiemann Reaction
Phenol on reaction with CHCl3, NaOH gives salicylaldehyde.
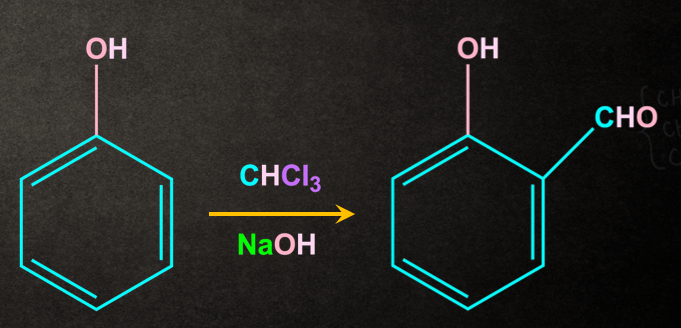
Mechanism of Reimer‒Tiemann Reaction:
Step 1: Formation of carbene
Reaction of trichloromethane with KOH in presence of heat gives an intermediate dichlorocarbene.
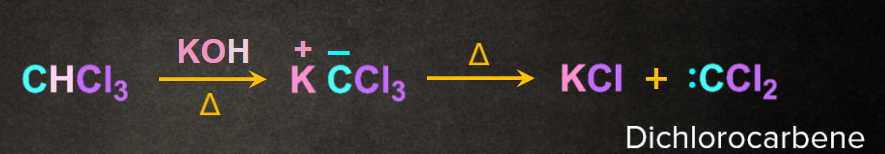
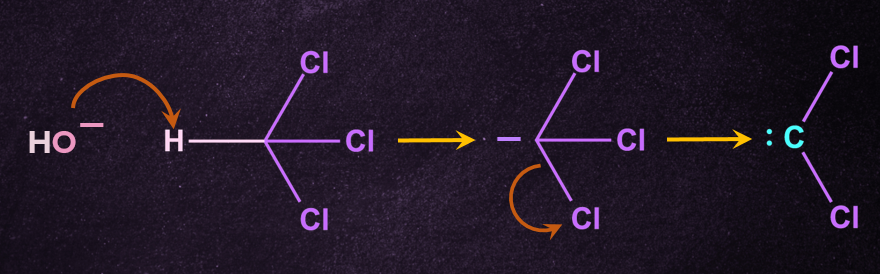
from KOH abstract a proton from the CHCl3 to form carbanion followed by removal of -Cl to form carbene intermediate.
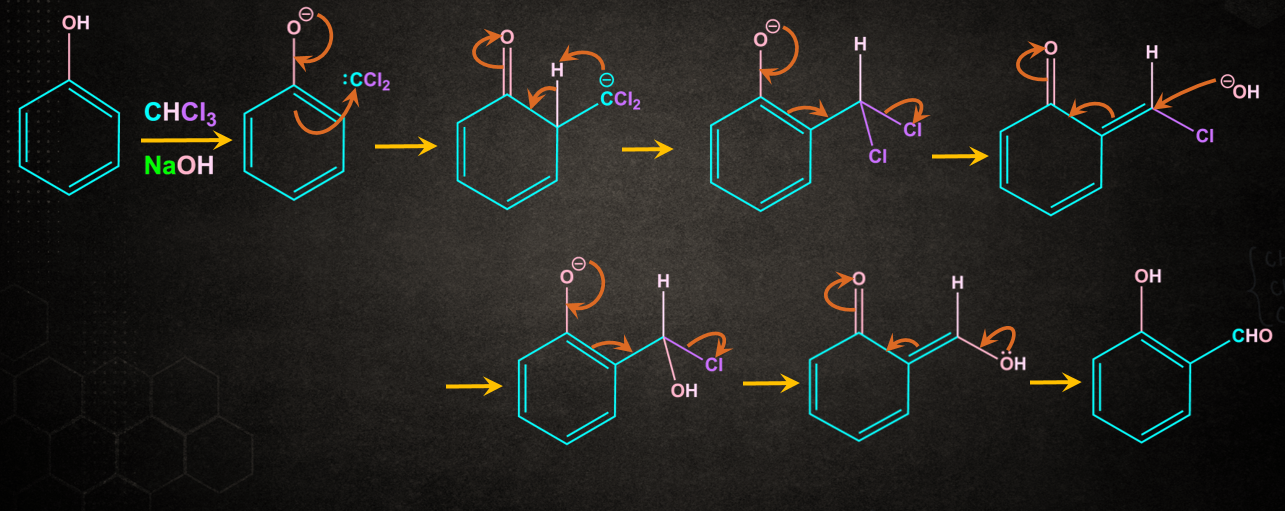
Step 2: Attack of carbene on phenol
Carbene gets attached to the ortho position of the phenol to form a non-aromatic intermediate. Proton (H+) gets removed to form an ortho-substituted phenoxide ion. Further rearrangement occurs to give salicylaldehyde.
elimination:
In elimination, two atoms/substituents/groups are removed from the adjacent atoms ( and positions) to form a new multiple bond. It is also known as 1,2- elimination.
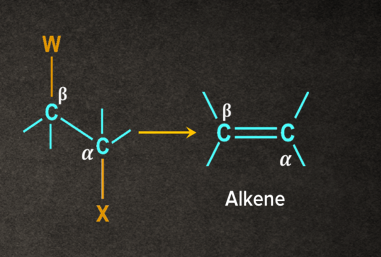
For example, when a haloalkane is made to react with a base, , in the presence of heat (, the elimination of the halogen (-X) from the -carbon and proton ( from the carbon takes place to give the corresponding alkene.
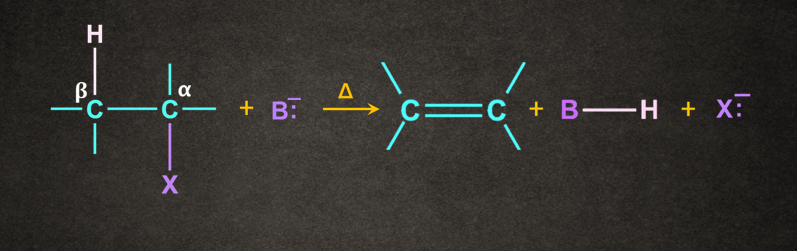
Mechanism of -elimination:
Generally, the base ( B-) abstracts a proton ( H+) from the followed by the removal of halide ionfrom the . This can be understood with the help of the mechanism given below.
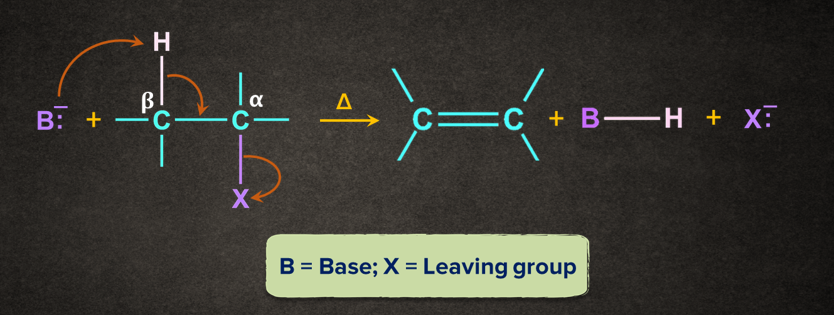
One of the important examples of elimination is dehydrohalogenation. In dehydrohalogenation, hydrogen atom from the 𝛃-C and a halogen from the 𝝰-C gets eliminated when heated with an alcoholic solution of KOH.

Types of 𝛃-elimination reaction:
Based on the fact that either abstraction of a proton or the removal of halogen is the first step or there will be simultaneous removal, elimination reactions can be classified into four types.
1. E1 elimination
2. E2 elimination
3. E1cB elimination
4. Ei elimination
1. E1 elimination:
In E1 elimination, the leaving group leaves the substrate first to form a carbocation intermediate. Then, the proton abstraction takes place to form an alkene. E1 elimination can be explained in detail by the following reactions.
Example- Dehydrohalogenation of an alkyl halide in the presence of water, and dehydration of alcohols in acidic medium are the examples of E1 elimination.
1. Dehydrohalogenation of an alkyl halide in the presence of water:
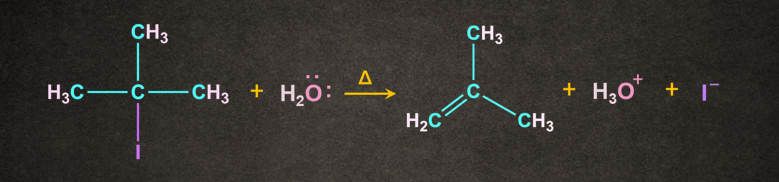
Mechanism of Dehydrohalogenation of an alkyl halide:
The reaction occurs in the presence of a weak base like H2O. It is a two-step process. Let's consider a reaction of 2-Iodo-2-methylpropane with H2O in the presence of heat. The reaction occurs in two steps as follows:
Step 1: Formation of carbocation
I being electronegative leaves as I- to form a tertiary carbocation.
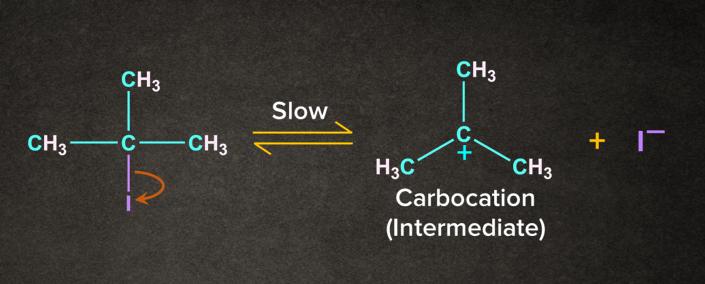
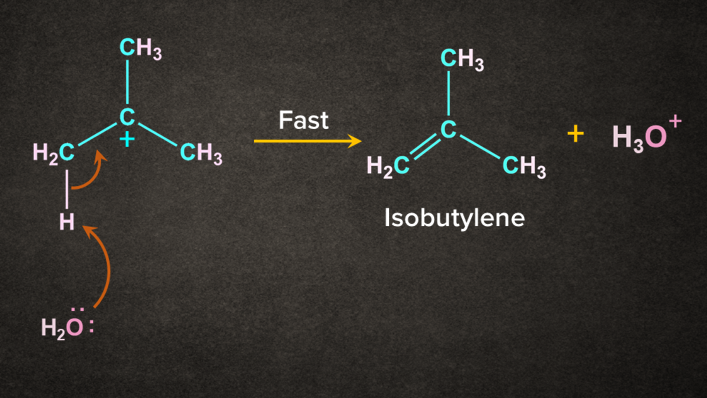
Step 2: Attack of base
H2O being a weak base abstract the H+ from the in the presence of heat to give 2-methylpropene (isobutylene).
2. Dehydration of alcohols in acidic medium:
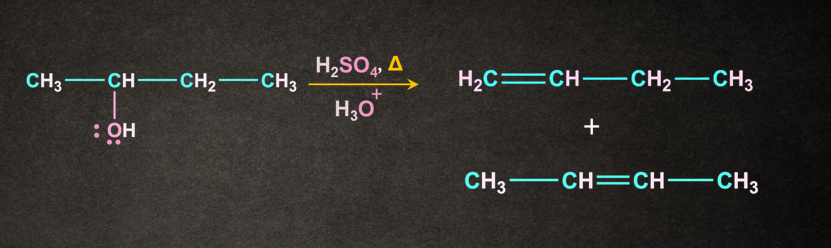
Mechanism of Dehydration of Alcohol:
Let us consider the dehydration reaction of butan-2-ol.
The reaction occurs in three steps.
Step 1: Protonation of alcohol
Oxygen being electron rich accepts the H+ to give protonated alcohol.
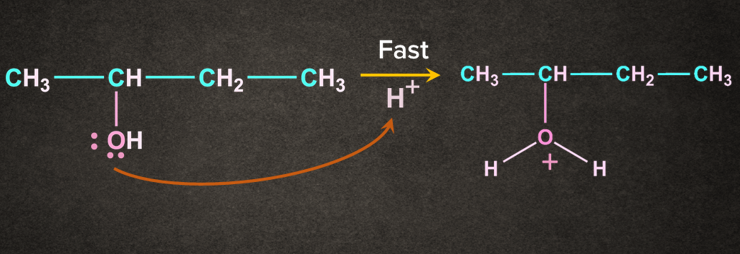
Step 2: Loss of leaving group
Protonated alcohol removes a water molecule to give carbocation. The carbocation formed here is a stable secondary carbocation.
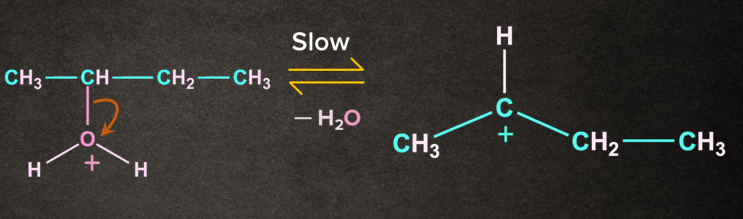
Step-3: Deprotonation to form alkene
There is the removal of to give an alkene. There are two types of available i.e. a and b.
Case 1: Removal of proton ‘a’ gives but-1-ene
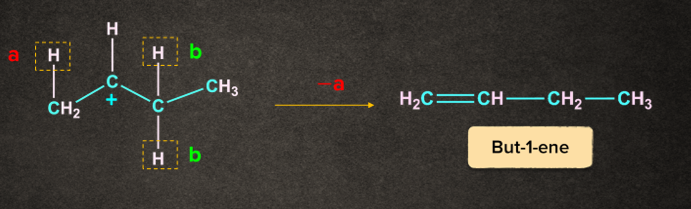
Case 2: Removal of proton ‘b’ gives but-2-ene
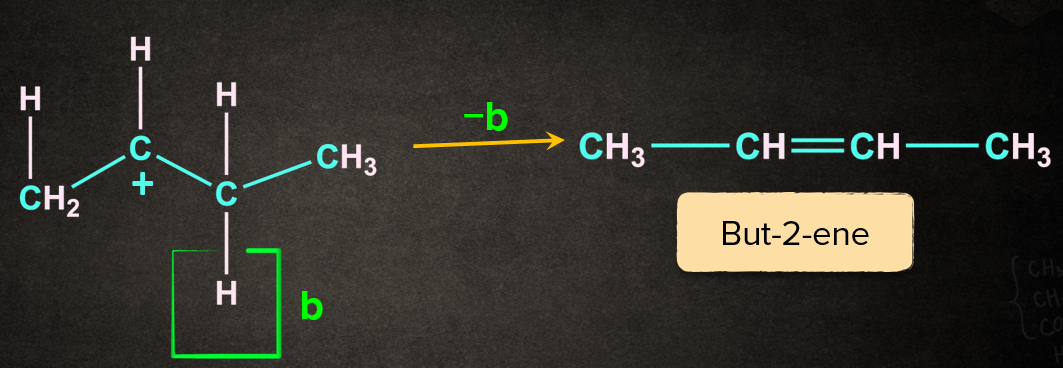
The reaction results in the formation of two products. The major product is decided with the help of Saytzeff's rule. According to this rule, the most substituted alkene is the major product. Generally, it is the most preferred one. But-2-ene being the most substituted out of the two will be the major product.
2. E2 elimination:
Generally, two groups/atoms/substituent depart simultaneously from adjacent carbons along with the proton being abstracted by a base.
Example- Dehydrohalogenation of alkyl halide in presence of base, (RONa)
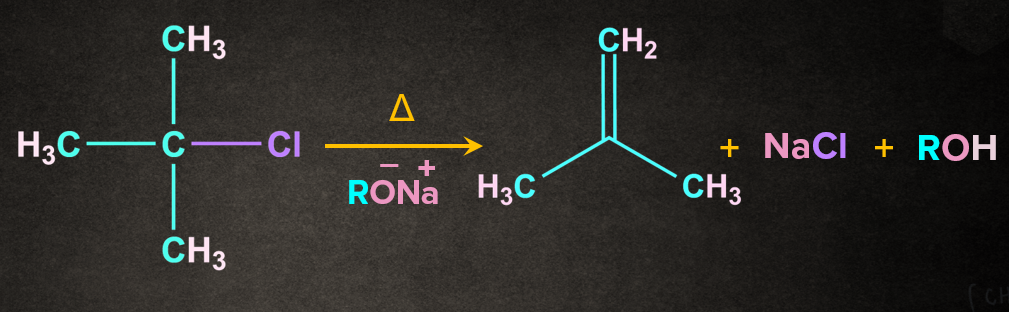
Mechanism of Dehydrohalogenation of alkyl halide by E2 mechanism:
As the name dehydrohalogenation suggests, halogen and hydrogen are removed. The reaction occurs in the presence of a strong base like . E2 reaction occurs in one step through a transition state. Let's consider a reaction of 2-chloro-2-methylpropane with in the presence of heat. It is a one-step process.
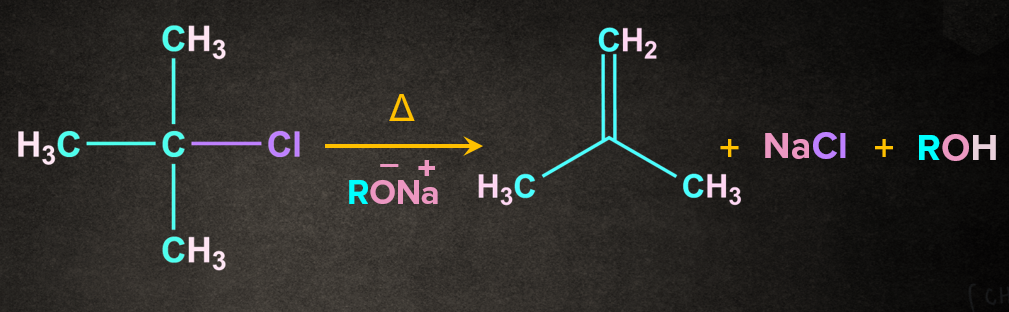
Firstly, RO- abstracts a proton from the followed by the simultaneous removal of Cl- to give 2-methylpropene. During the abstraction of the proton by the base and the removal of the leaving group, a transition state is formed. A partial double-bond character is observed in the transition state.
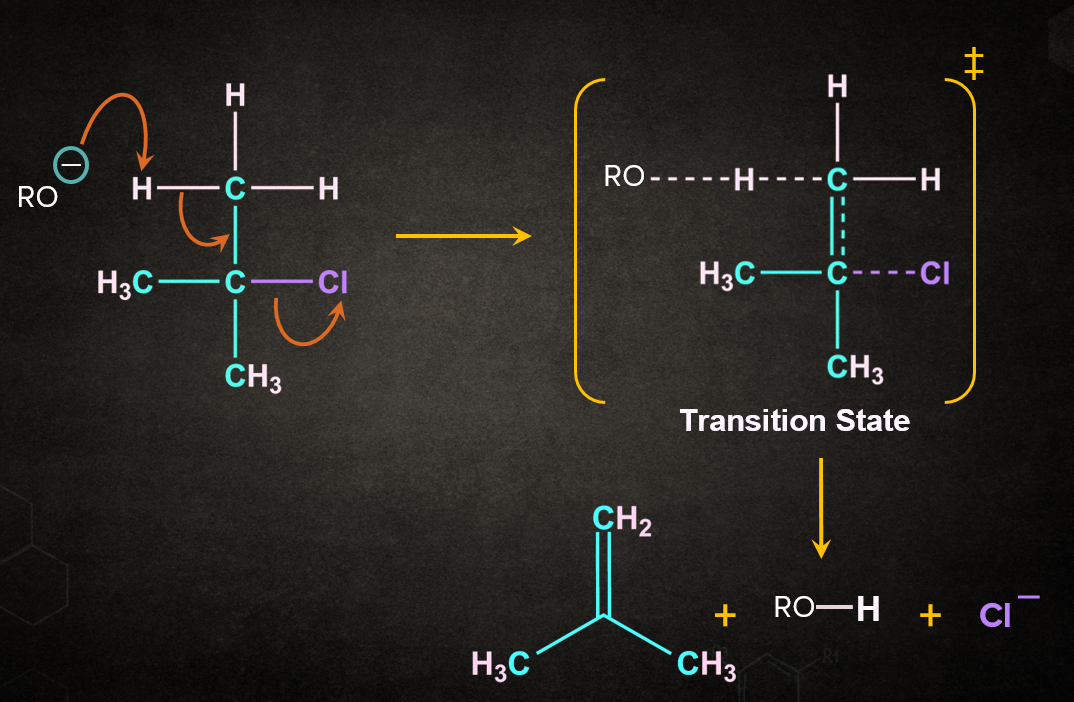
E2 reaction is a concerted reaction because the bond formation and the bond-breaking steps take place simultaneously. A concerted reaction is one in which the abstraction of a proton by a base and the departure of the leaving group occur simultaneously.
3. E1cB Elimination:
In E1cB, E stands for elimination, 1 for unimolecular, and cB for conjugate base. In E1cB, the proton is abstracted to form the conjugate base. The anion that results is stable enough to exist because it can
be delocalised on to the electron-withdrawing group. Although the anion is stabilized by the electron-withdrawing group, it still prefers to lose a leaving group and become an alkene.
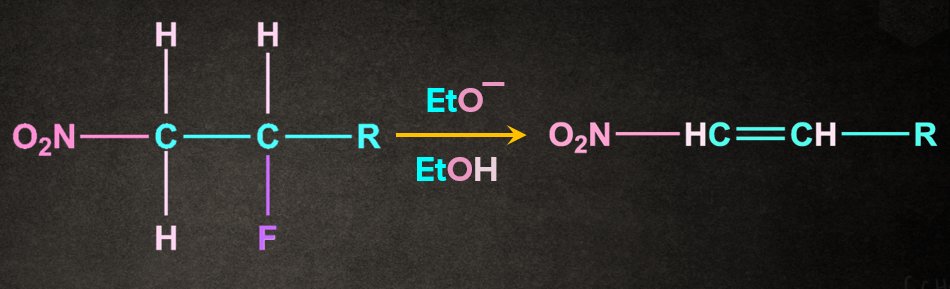
Conditions for E1cB Elimination:
There are two conditions for any molecule to give E1cB elimination reaction:
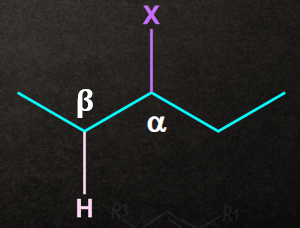
1. A good electron withdrawing group must be present at the β-position to the leaving group.
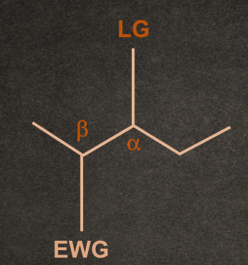
Example- Carbonyl (-C=O), nitro (-NO2), cyano (, sulphonyl (-SO2-),
Phenyl (-Ph), ester (-COOR), and other carbonyl stabilizing groups.
2. Generally, the poor leaving group shows E1cB reaction.
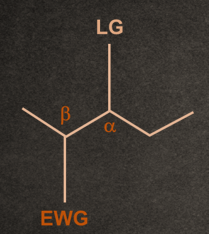
Example:
Mechanism of E1cB Elimination:
The mechanism of E1cB reaction is a two-step process.
Step 1: Abstraction of proton to form carbanion
Base, EtO- abstracts the proton from the carbon to which electron withdrawing group, nitro(-NO2) is attached. The removal of the proton gives the carbanion intermediate.
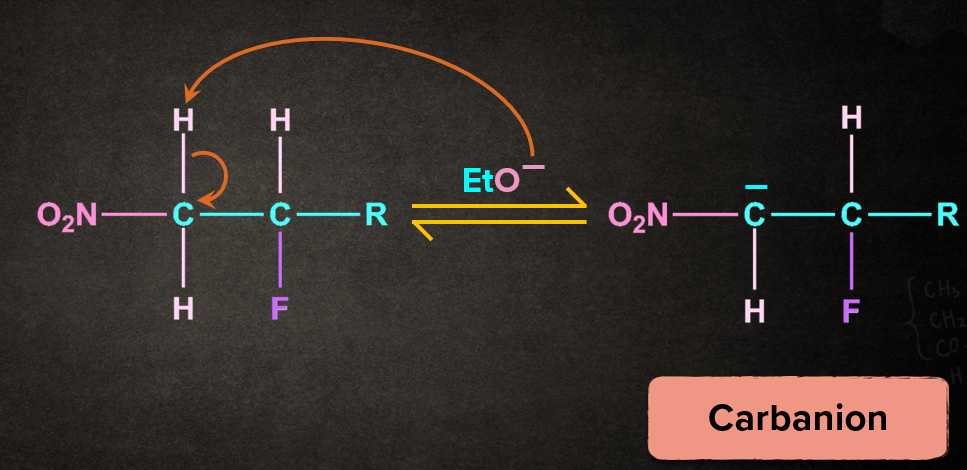
Step 2: Removal of leaving group to give an alkene
Leaving group, attached to the adjacent carbon of the carbon containing negative charge, leaves such that a π bond is formed between the two carbons containing the leaving group and negative charge.
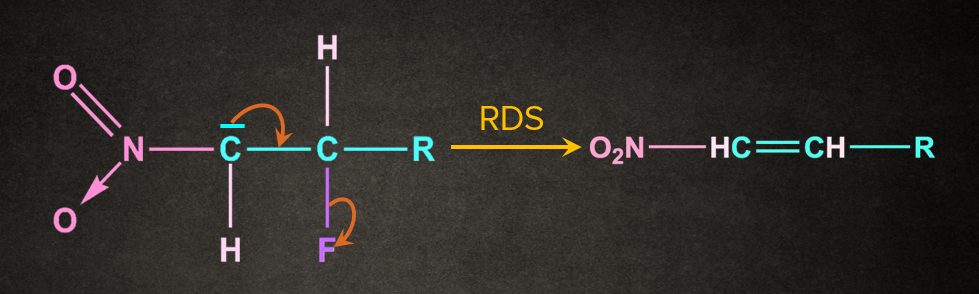
4. Ei Elimination reaction:
It is a class of elimination reactions in which a compound undergoes elimination on heating, with no other reagent present.
Example- In the below given reaction, a molecule gets eliminated from the reactant by simply heating the reactant at

The mechanisms are different from those already discussed elimination reactions since all those require a
base (which may be the solvent) in one of the steps. It follows unimolecular, concerted mechanism where two groups leave at about the same time and bond to each other simultaneously. Thus, involves a five, or six-membered cyclic transition state.
Ei elimination is also known as syn elimination because two groups leave and bond to each other simultaneously when they are syn to each other. It is also known as Pyrolytic elimination because it occurs under thermal conditions.
Condition for compounds showing Ei Elimination:
Compounds containing at least one β-hydrogen atom shows Ei Elimination reaction.
Example- Esters, xanthate esters, 3o amine oxide undergoing Ei elimination reactions upon heating are shown below.
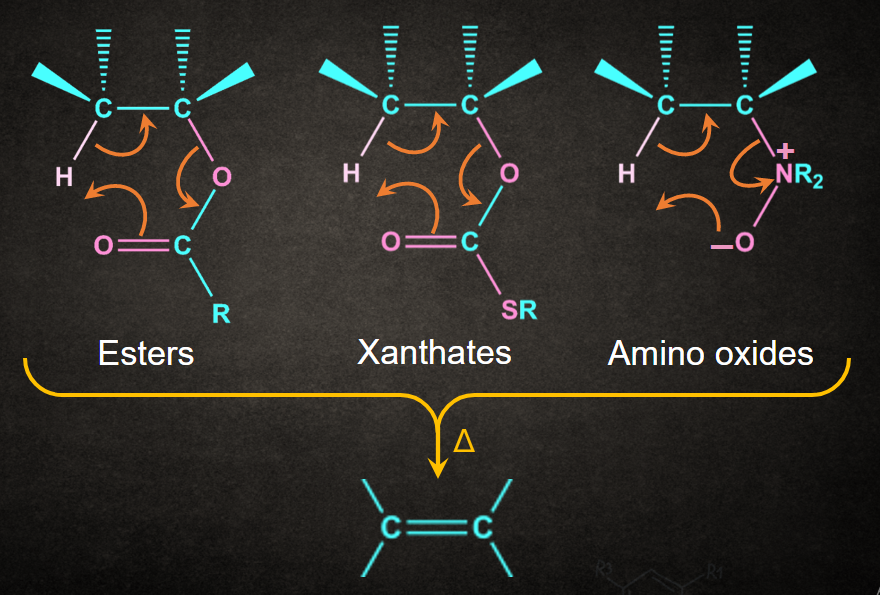
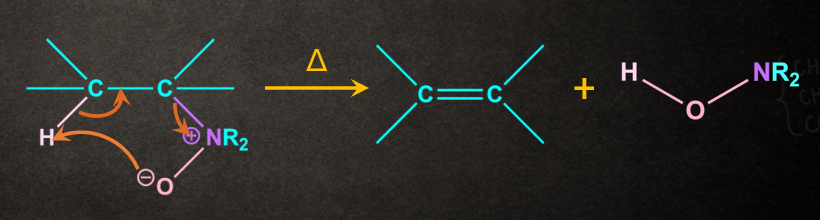
Note: Cope Elimination is an example of Ei elimination.
In Cope elimination, amine oxide undergoes Ei elimination via a concerted mechanism through a cyclic transition state.
Related video: Elimination reactions
Practice Problems:
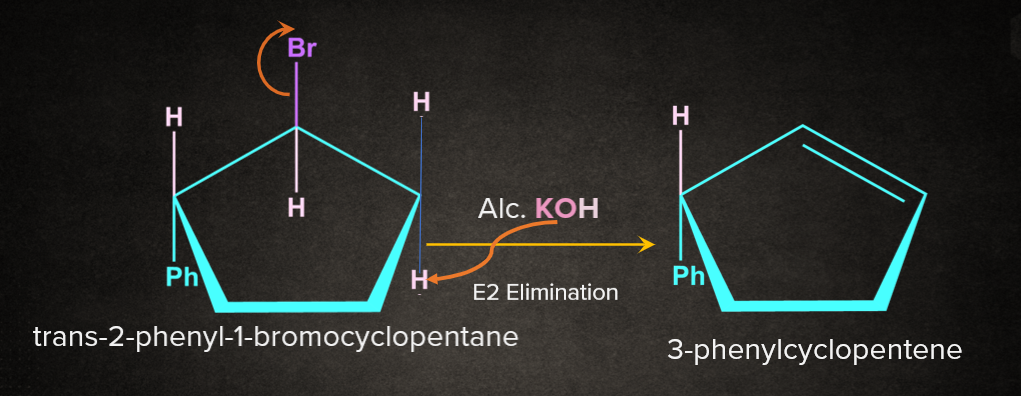
Q1. The reaction of trans-1-bromo-2-phenylcyclopentane on reaction with alcoholic KOH produces:
A. 4-phenylcyclopentene
B. 2-phenylcyclopentene
C. 1-phenylcyclopentene
D. 3-phenylcyclopentene
Answer: D
Solution: The reaction of trans-1-bromo-2-phenylcyclopentane on reaction with alcoholic KOH is an E2 elimination reaction. In an E2 reaction, the base removes the proton from the alkyl halide that is oriented anti to the leaving group (), and the leaving group leaves simultaneously. The removal of the proton by the base, the removal of the leaving group and the formation of the double bond all happen in one concerted step. The product formed is 3-phenylcyclopentene.
So, option D) is the correct answer.
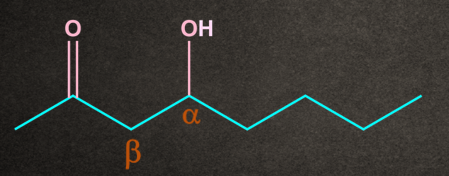
Q. 2.
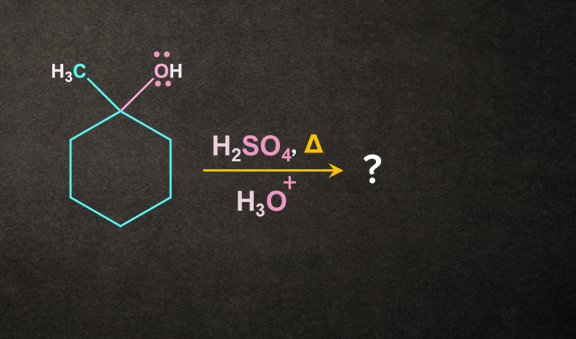
The major product formed will be:
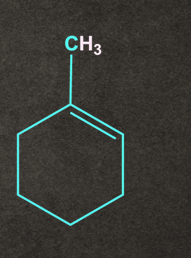
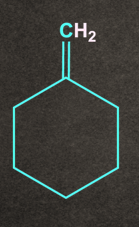
- Both A and B
- None of the above
Answer: A
Solution:

According to the Saytzeff rule, A being the more substituted alkene, will be the major product.
So, option A) is the correct answer.
Q3. What are the essential conditions for any reaction to show E1cB mechanism?
A. Presence of electron withdrawing group
B. Presence of electron donating group
C. Poor leaving group
D. Both A and C
Answer: D
Solution: There are two conditions for any molecule to give E1cB elimination:
1. A good electron withdrawing group must be present at the β-position to the leaving group.
2. Presence of poor leaving group.
Q4. Which of the following substrates undergo elimination via E1cB mechanism?
A. 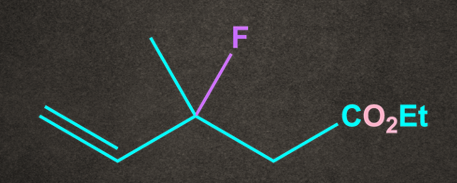
B. 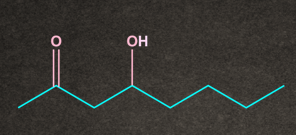
C. 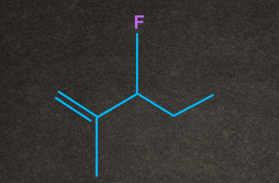
D. Both A and B
Answer: D
Solution: There are two conditions for any molecule to give E1cB elimination:
1. A good electron-withdrawing group must be present at the β-position to the leaving group.
2. Presence of poor leaving group.
Both A and B have electron-withdrawing groups at as well as the poor leaving group at . So both the conditions of the E1cB mechanism are satisfied.
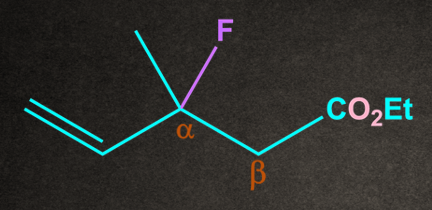
Therefore, options A and B undergo the E1cB mechanism.
Frequently asked Question-FAQs:
Q1. What is carbene?
Solution: Carbenes are neutral reaction intermediates having divalent carbon which has two valence electrons distributed among two nonbonding orbitals.
Characteristics of Carbene:
1. Carbene is highly reactive reaction intermediate
2. It is highly unstable
3. It is an electron deficient
4. Carbene is divalent in nature
Q2. What are the types of carbene?
Solution: Carbenes can be classified into two types:
(1) Singlet carbene
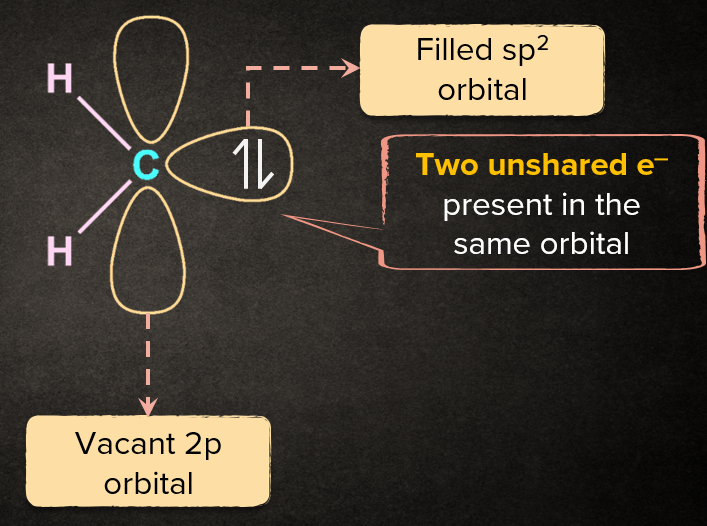
(2) Triplet carbene:
Q3. Why does the dehydration of alcohol take place in an acidic medium but not in a basic medium?
Solution: In an acidic medium, -OH gets converted into which is a good leaving group. In a basic medium -OH is the leaving group, and it is a poor leaving group. So, the dehydration of alcohol generally takes place in an acidic medium.
Q4. What is a ?
Solution: Carbon that is directly attached to the carbon which is attached to the functional group, is called n.