-
Call Now
1800-102-2727
Electrophilic Addition Reactions on Alkenes: Addition of X2, HOX, HX, H2O, Practice Problems, FAQs
Let us perform an experiment!
In three test tubes, we put hexane (C6H14), hexene (C6H12), and hexyne (C6H10). The reddish-brown colour of Br2 / CCl4 is retained when it is added to hexane. When Br2 / CCl4 is added to hexene, however, the reddish-brown colour is disappeared (decolorised), and 1,2-dibromohexane is produced. When Br2 / CCl4 is added to hexyne, the reddish-brown colour disappears (decolorizes), and 1,1,2,2-tetrabromohexane is produced.

Why is that so that the reddish-brown colour of Br2 / CCl4 is retained and decolourisation occurs in the cases of alkenes and alkynes? Can you think of the basic difference between alkanes and alkenes/alkynes? Clearly, alkanes are saturated compounds and alkenes/alkynes are unsaturated compounds. Alkenes are known to be electron-rich because of the loosely held π electrons. And we know that any electron-rich moiety will try to react with electron-deficient moiety. So is the case with alkenes, Alkenes being electron rich have a tendency to react with electron-deficient species(electrophiles).
So let's study in detail what all such reactions alkene give!
Table of Content
- Introduction
- Addition of X2
- Addition of Hypohalous Acid, HOX
- Addition of hydrogen halide, HX
- Addition of water, H2O
- Practice Problems
- Frequently Asked Questions-FAQs
Introduction
Alkenes are rich sources of loosely held π electrons. Electrophilic addition reactions are given by electron-rich species like alkene. They have a tendency to attract electron-deficient species like electrophiles towards them. Therefore, whenever they react with species like HCl, Br2, etc. the first step which is the rate-determining step involves the attack of the electrophile, H+, Br+ respectively. Due to this reason, they are known as electrophilic addition reaction.
Addition of halogens, X2
Halogens like bromine and chlorine add up to alkene to form vicinal dihalides.
Example- Addition of Cl2 to ethene in presence of CCl4 as solvent.
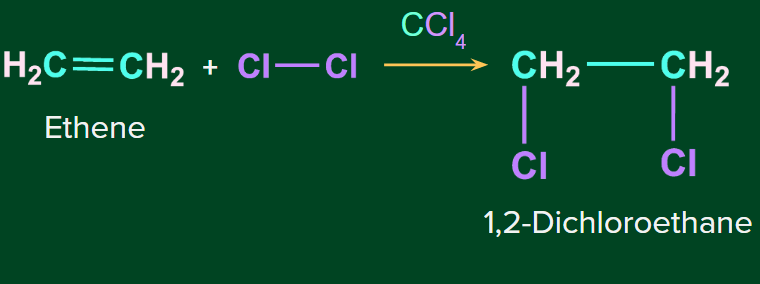
Note: This reaction can be used as a test for unsaturation.
Test for unsaturation with Br2 in CCl4
The reddish-brown color of bromine solution in carbon tetrachloride disappears when bromine adds up to an unsaturated site. Hence, this reaction is used as a test for unsaturation. Alkenes decolourise the reddish-brown color of Br2 / CCl4 . This test is also known as Baeyer's test.
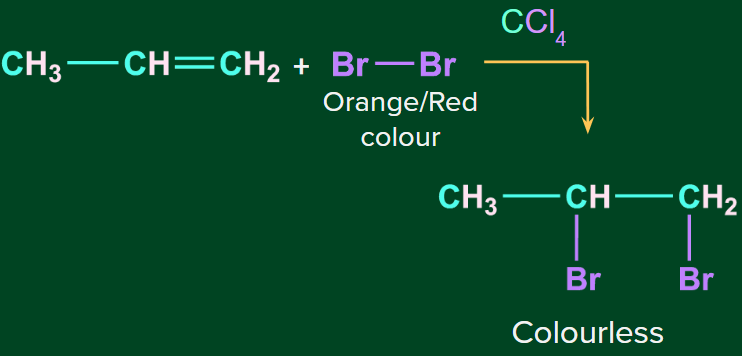
Note: Alkanes on the other hand don’t give this test.
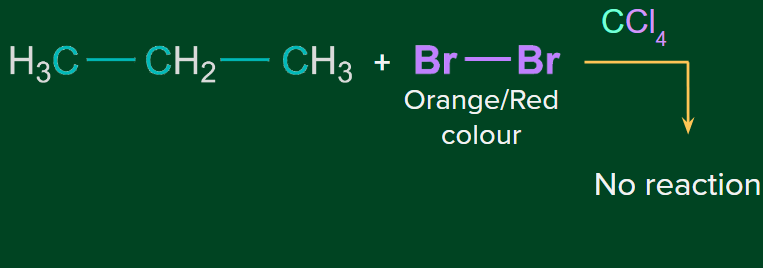
- Iodine doesn't show addition reaction under normal conditions.
- Fluorine doesn't show addition reaction under normal conditions because F+ is never formed.
Mechanism of addition of X2
The addition of halogens to an alkene is an example of an electrophilic addition reaction involving cyclic halonium ion formation.
There are two steps involved in the addition of halogens.
Step 1- Formation of Bromonium ion
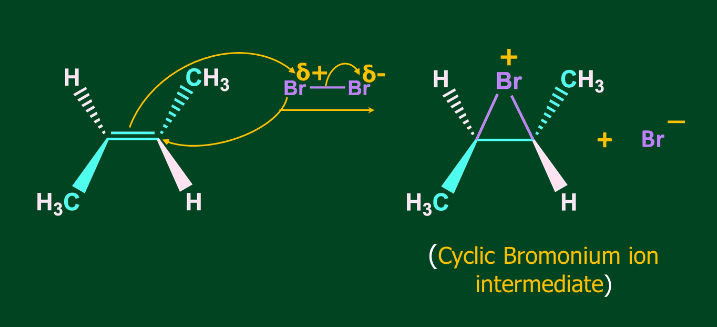
Step 2- Attack of Br- on Bromonium ion
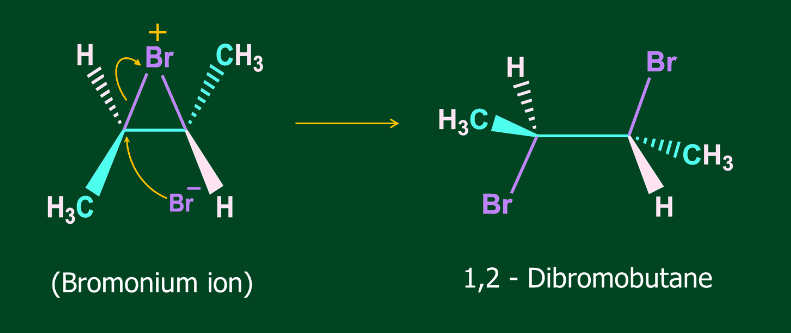
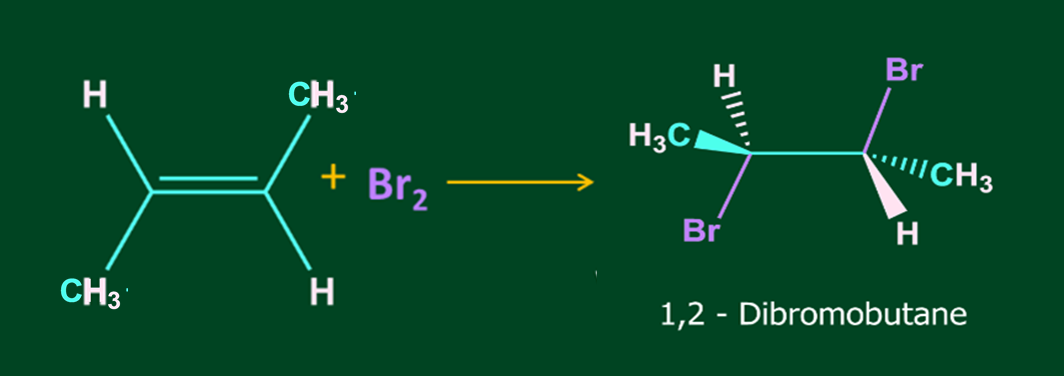
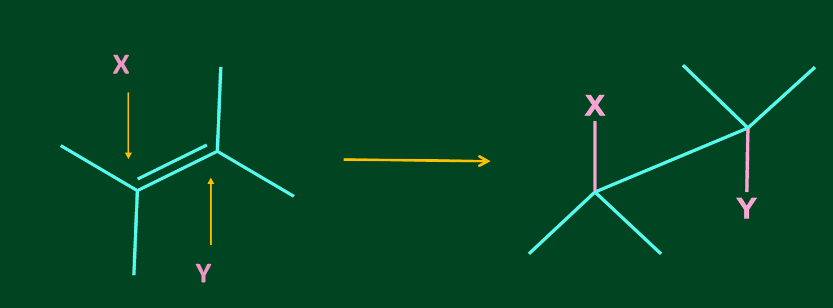
Addition of X2 to alkene is an anti-addition i.e. addition of two substituents to multiple bonded C atoms from the opposite sides of the multiple bond.
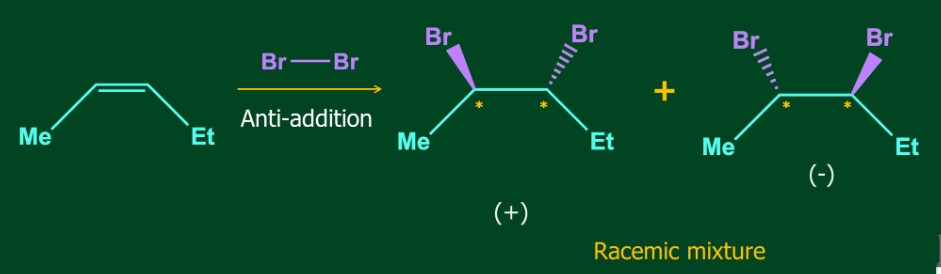
Halogen addition to alkenes generates only a racemic mixture if provided terminals of the double bond are different.
Addition of Hypohalous Acid( HOX)
Addition of HOX to alkene occurs in presence of of CCl4 gives corresponding chloroalcohol.
Example- Reaction of ethene with HOCl in presence of CCl4 gives 2-Chloroethanol.
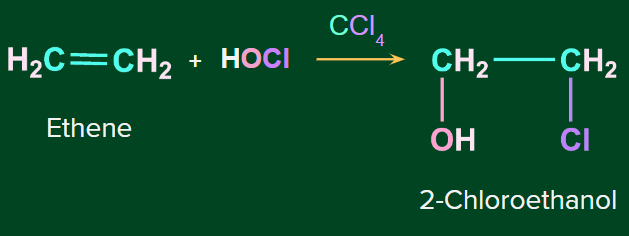
The general reaction is represented as follows:
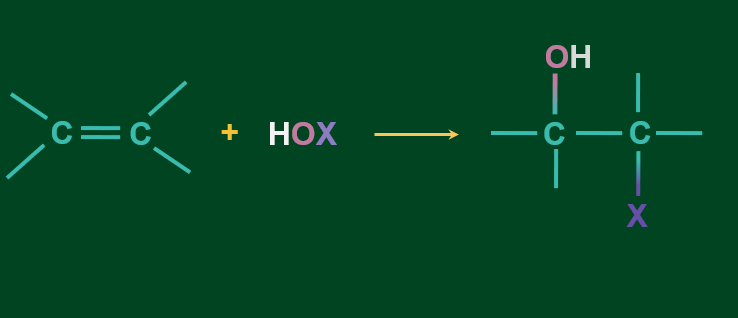
Mechanism of addition of HOX to alkene
Step 1: HOX cleaves as X+ and OH- and X+ attacks the alkene to form cyclic intermediate.
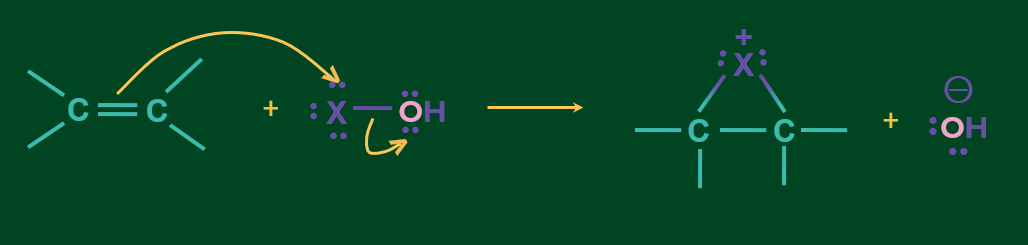
Step 2: C-X bond cleaves followed by the attack of OH- on the carbon. Further deprotonation occurs to chloroalcohol.
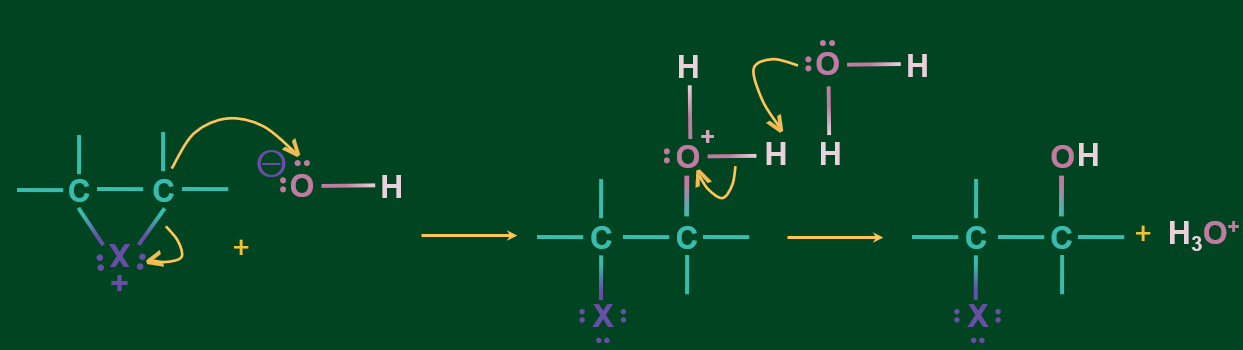
Note: If we take unsymmetrical alkene then, -OH gets attached to the more substituted C of the alkene and -Br gets attached to the less substituted C of the alkene.
Addition of Hydrogen Halides (HX)
Alkenes react with hydrogen halide in presence of a solvent like CCl4to give corresponding alkyl halide.
Example- Addition of HBr to propene.
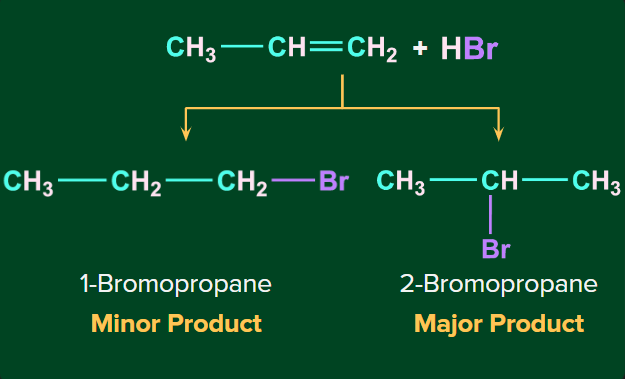
Addition of HX on unsymmetrical alkene occurs via Markovnikov’s rule.
According to Markovnikov’s rule, anion (X-) majorly gets attached to that C-atom where the carbocation intermediate is more stable.
Lets us consider the reaction of unsymmetrical alkene, 3-Methylbut-1-ene with HBr to give 2-Bromo-2-methylbutane as the major product.
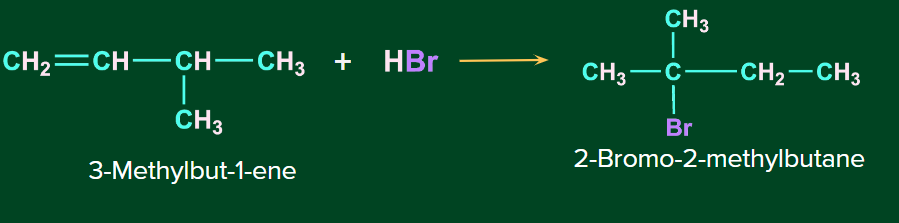
The first step involves the attack of electrophile, H+ on the C1 carbon of the alkene to give secondary carbocation.
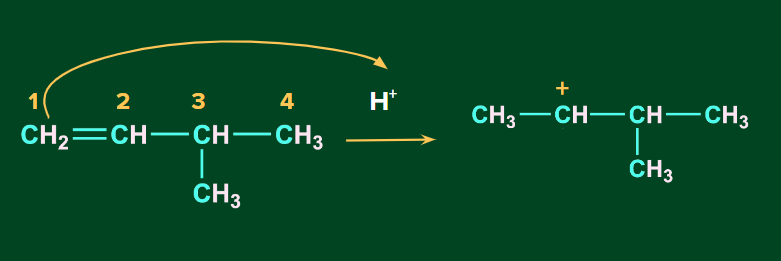
Secondary carbocation being unstable, undergo a 1-2-hydride shift to form a more stable tertiary carbocation.

Finally, anion Br- gets attached at the tertiary carbocation to give 2-Bromo-2-methylbutane as the major product.
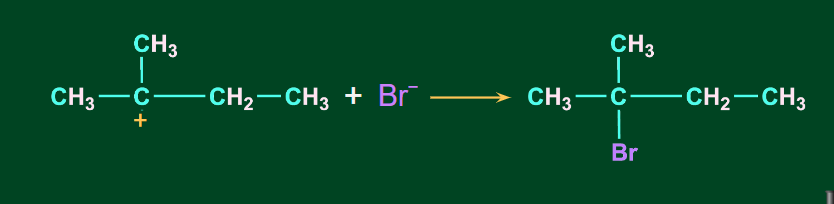
Order of reactivities of different alkenes is directly proportional to the stabilities of the carbocations formed from alkenes.
Benzyl carbocation > Allylic carbocation > 30 carbocation>20 carbocation > 10 > Carbocation
Also the rate of reaction depends upon the reactivities of hydrogen halides(H-X).
H-F < H-Cl < H-Br < H-I
![]()
With increasing the bond lengths, bond strength decreases, as a result of which reactivity of H-X increases.
Addition of Water (H2O)
There are the following ways in which water can be added to an alkene to show an electrophilic addition reaction.
- Acid-catalyzed Hydration
- Oxymercuration-Demercuration Reaction
Acid-catalyzed hydration:
This is also known as the hydration of alkenes. The addition of H2O/H+(acid catalyzed hydration) to alkenes is a Markovnikov addition reaction.
Example: Addition of H2O to 2-Methylpropane.
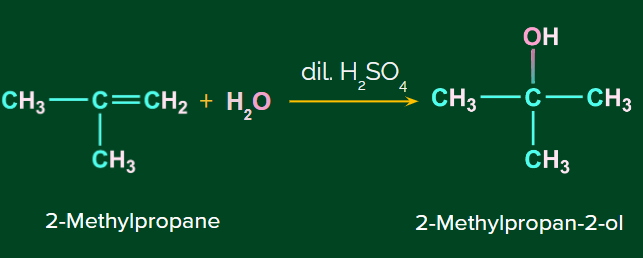
Mechanism of Acid catalyzed hydration:
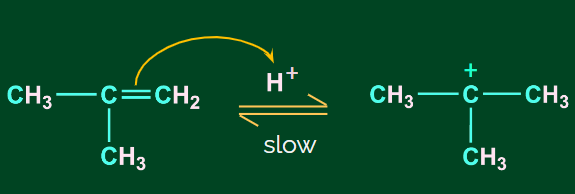
Step 1: Protonation and formation of carbocation
When the protonation of a double bond occurs it breaks, it forms a stable carbocation. Here, a tertiary carbocation is formed instead of a primary carbocation. This is because the tertiary carbocation is more stable than the primary carbocation.
Step 2: Nucleophilic attack of H2O
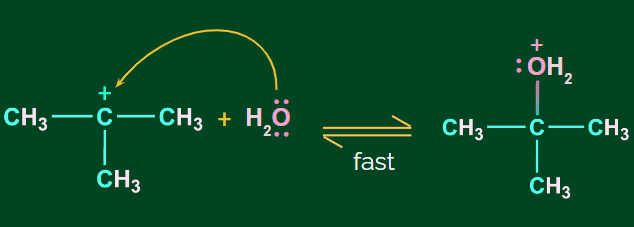
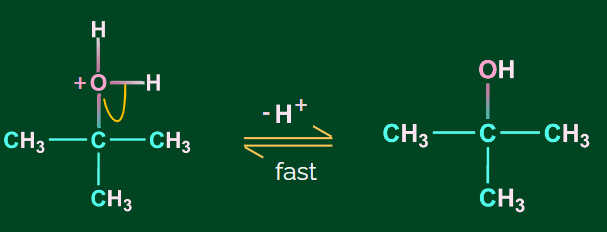
Step 3: Deprotonation to form alcohol
Some other examples of hydration of alkene in presence of acid are given as follows:
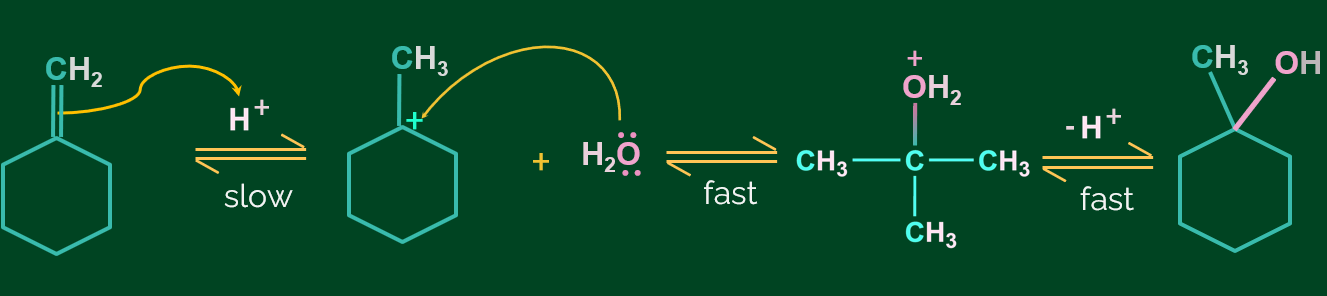
Hydration by Oxymercuration-Demercuration:
Alkenes can be converted to alcohol using the oxymercuration-demarcation reaction. An alkene is treated with mercuric acetate in a Tetrahydrofuran–water solution to produce a product, which can then be reduced with NaBH4 to produce alcohol. Markovnikov's rule operates in this reaction.
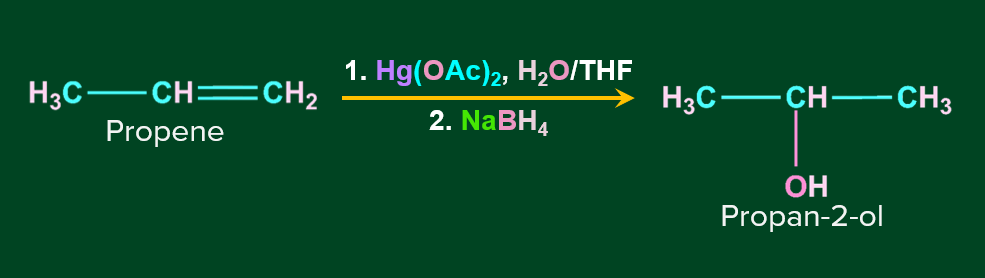
Mechanism of Oxymercuration-Demercuration
The mechanism of this reaction follows Markonikov's regioselectivity rule, with the hydroxyl group joining the more substituted carbon atom of the double bond and the hydrogen atom joining the least substituted carbon atom of the double bond.
The reaction mechanism is depicted below.
Step 1: In this step, the nucleophile C=C bond attacks -Hg, by the simultaneous removal of the acetate ion. This results in the formation of cyclic mercurinium ion, intermediate.
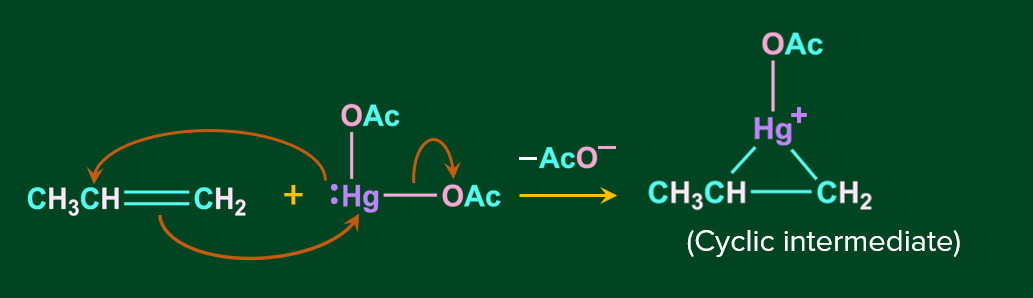
Step 2: In this step, the nucleophile attacks the most substituted carbon, according to the markovnikov’s rule, resulting in the cleavage of the corresponding C-Hg bond.
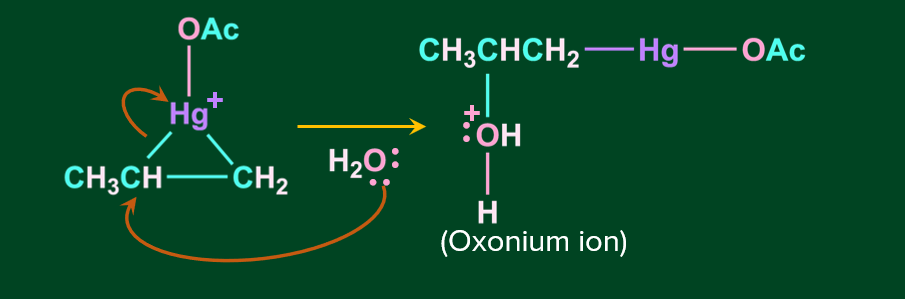
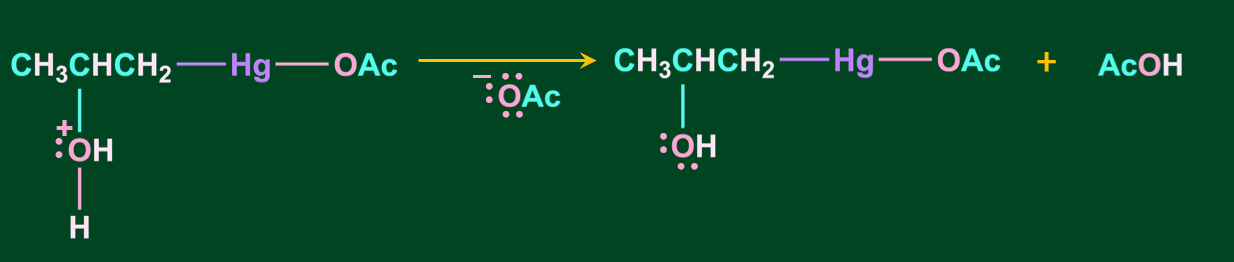
Step 3: In this step, the oxonium ion is deprotonated in the presence of acetate ion( which acts as a base) resulting in alcohol.
Step 4: In the final step, upon the addition of sodium borohydride, NaBH4 the acetyl mercury(-Hg-OAc) gets replaced with a hydrogen atom, resulting in the formation of alcohol via a new C-H bond.
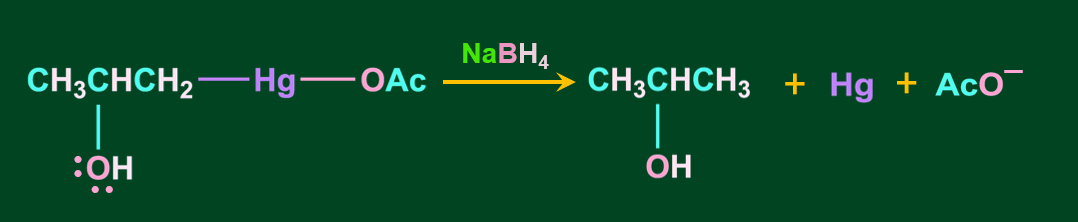
Practice Problems
1. In the given reaction, what will be the major product?
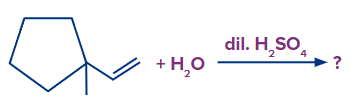
- 2-(1-methylcyclopentyl)ethan-1-ol
- 1,1-Dimethylcyclohexan-2-ol
- 2,2-Dimethylcyclohexan-1-ol
- 1,2-Dimethylcyclohexan-1-ol
Answer: D
Solution:
Step 1: The first step is the protonation of the alkene, which results in the formation of a secondary carbocation. However, in this case, the carbocation formed results in the ring expansion and forms a six-membered stable ring.
Step 2: The addition of OH-occurs and the product formed is shown as follows:
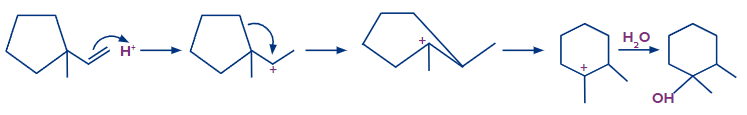
After rearrangement and addition of H2O, the final product is 1,2-dimethylcyclohexan-1-ol.
Hence, a correct answer is an option (D).
2. What is the order of reactivities of the following compounds with HBr?
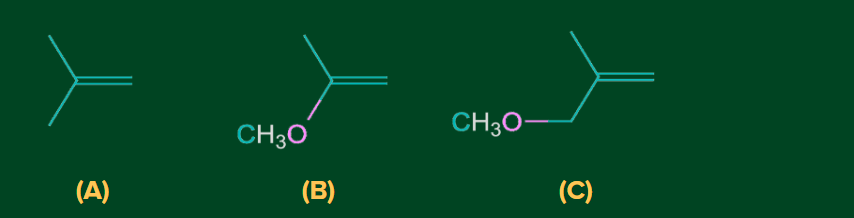
A) A > B > C
B) B > A > C
C) C > B > A
D) B > C > A
Answer: B
Solution: (A) A tertiary carbocation is formed which is stabilised by hyperconjugation of three -CH3 groups.
(B) Methoxy group(-OCH3) stabilises the tertiary carbocation via mesomeric effect(+M effect).
(C) Methoxy group(-OCH3) destabilises the tertiary carbocation via –I effect of O atom.
Therefore out of three carbocation of compound (B) will be the most stable, followed by (A) and then the least stable would be of (C).
So, option (B) is correct.
3. C6H5CH=CHCH3 reacts with HBr to form:
A) 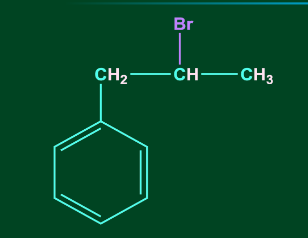
B) 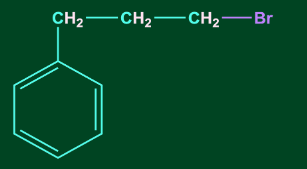
C)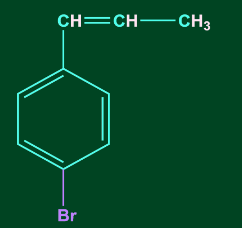
D) 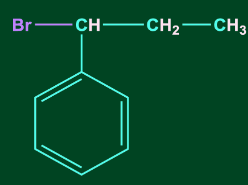
Answer: D
Solution: Addition of HX to unsymmetrical alkene occurs via Markovnikov’s rule.
According to Markovnikov’s rule, anion (X-) majorly gets attached to that C-atom of the double bond where the carbocation intermediate is more stable. Therefore, benzyl carbocation will be the most stable carbocation formed in this case. Hence option, D is the correct answer.
4. In which of the following reaction cyclic intermediate is formed?
A) Electrophilic addition of HOCl to alkene
B) Electrophilic addition of HBr to alkene
C) Electrophilic addition of Hg(OAc)2 to alkene
D) Both A and C
Answer: D
Solution: Electrophilic addition of HOCl and Hg(OAc)2 to alkene proceeds via formation of cyclic chloronium and mercurinium ion intermediate respectively.
Frequently Asked Questions
1. What applications does alkene have?
Answer: They are used in the production of plastics such as polythene for buckets, bowls, and bags and polystyrene for car battery cases and refrigerator parts. They are used in the production of ethane-1,2-diol, which is used as an anti-freeze agent in automobile radiators.
2. What are the applications of the Hydroboration-oxidation and Oxymercuration-Demercuration reactions?
Answer: It is used in the production of alcohol from alkenes. It produces more stereospecific and regioselective alcohols than other oxidation reactions used for the alcohol formation.
3. Why does carbocation rearrange itself?
Answer: Stability of the product depends upon the stability of carbocation intermediate. Therefore the more the stable intermediate, the greater the stability of the product formed. That’s why carbocation rearranges itself to form a more stable one.
4. Can benzene decolourise bromine water?
Answer: Benzene does not show an addition reaction with bromine so it does not decolourise bromine water.


