-
Call Now
1800-102-2727
Effect of Catalyst on the Rate of a Reaction- Catalyst, Characteristics, Types, Effect on the Rate of a Chemical Reaction, Practice Problems, FAQs
I'm sure that most of you see the international cricket matches. But have you ever been to a stadium to watch an international cricket match? Does watching in the stadium directly of any advantage?
Watching a cricket match by going to the stadium, gives a more authentic real-time experience. Both spectators and the players feel the full experience and emotions exchanged between them instantly..
Spectators can support their favourite teams and players, display banners, and enjoy the vibrant and energetic atmosphere that pervades the cricket stadium.
Not only the spectators, but the audience also influence the cricket players.
The stadium audience acts as a catalyst, helping to boost the morale of the home team and thus helping them win the game.
Some similar phenomena are observed in chemistry in which some compound helps the reaction to complete faster and is known as a catalyst.
Gottlieb Kirchhoff, a Russian chemist studied the use of an external substance in an organic reaction. A Swedish chemist named Jöns Jakob Berzelius used ‘catalysis’ to explain for the first time the changes in the rate of chemical reactions.
Let us learn more about the catalyst in this article.

Table of Contents
- Catalyst
- Characteristics of Catalysts
- Types of Catalysts
- Effect of Catalyst on Rate of Reaction
- Practice Problems
- Frequently Asked Questions-FAQs
Catalyst
Catalysts are defined in chemistry as substances that change the rate of a reaction by changing its path. A catalyst is typically used to accelerate or increase the rate of a reaction. On a deeper level, catalysts are used to break or rebuild the chemical bonds that exist between the atoms in the molecules of various elements or compounds.
Catalysts can be in any physical state as solid, liquid, or gas. Catalysts can be metal, metal oxide, sulphide, halide, etc. Semi-metal compounds of boron and silicon also act as catalysts.
A catalytic reaction is a reaction that involves an external substance (catalys) as one of the reactants to alter its rate from that of an uncatalysed reaction. The catalyst forms an intermediate with the substrate reactant which then decomposes to form the product. The intermediate formation requires lesser activation energy. The catalyst is regenerated after the completion of the reaction, perhaps in a different physical form.
Catalytic activity is a surface phenomenon and hence the activity of the solid catalyst is directly proportional to the active centres' on the catalyst which is again related to the surface area and crystal structure. Some solid catalysts may have different activity centres and hence can exhibit a polyfunctional catalytic activity
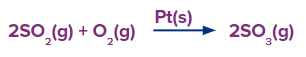
In the above reaction SO2 and O2are the reactants, reacting to form SO3 molecule. Pt(s) act as a catalyst in this reaction and increases the speed of reaction so that the reaction completes faster.
Characteristics of Catalysts
- A catalyst's mass and chemical composition remain unchanged during the reaction. Its physical state, on the other hand, is subject to change. For example, granular MnO2 is left as a powder at the end of the KClO3 decomposition reaction.
- The greater the surface area of the catalyst, the faster the reaction. To increase the rate of the reaction, finely divided catalysts are used.
- Catalysts are reaction-specific molecules. A substance that catalyses one reaction may not catalyse others.
- The efficiency of a catalyst is greatest at a specific temperature, known as the optimum temperature. The activity of the catalyst decreases at any temperature above or below the optimum temperature.
- Biological enzymes, too, only function at a specific pH. The pH at which enzyme activity is at its peak is referred to as optimum pH.
- The reaction cannot be started by a catalyst.
- A catalyst has no effect on the enthalpy of a reaction.
- A catalyst cannot alter the equilibrium or the equilibrium concentration. It can only change the rate of attainment of equilibrium.
Types of Catalysts
- On the basis of the phase of the catalyst and the phase of the reaction, catalysts are classified into two types:
- Homogeneous catalyst:
It is defined as the reaction in which the phase of the catalyst and the reactants used in the reaction are same. For example, let us consider a reaction,
oxidation of sulphur dioxide with oxygen, in presence of NO(g) as catalyst.
2SO2(g) + O2 (g) 2SO3(g)
In the above reaction, phase of reactants is the same as the phase of the catalyst used in the reaction. (i.e. all the reactants and catalyst is in a gaseous state).
Hydrolysis of sugar in acidic medium.
In this reaction, H2SO4(aq) is used as a catalyst
C12H22O11(aq) + H2O(l) C2H12O6(aq) +C6H12O6(aq)
In the above reaction, phase of reactants is the same as the phase of the catalyst used in the reaction. (i.e. all the reactants and catalyst is in an aqueous state).
- Heterogeneous catalyst:
It is defined as the reaction in which the phase of the catalyst and the reactants used in the reaction are different. For example, let us consider a reaction,
Decomposition of H2O2 in presence of MnO2(s), as a catalyst.
2H2O2(l) H2O(l) +O2(g)
In the above reaction, phase of reactant is not same as the phase of the catalyst used in the reaction. (i.e. reactant is in liquid state and catalyst is in solid state).
SO3 formation, in presence of Pt(s), as a catalyst.
2SO2(g) + O2 (g) 2SO3(g)
In the above reaction, the phase of reactants is not the same as the phase of the catalyst used in the reaction. (i.e. reactants are in a gaseous state and catalyst is in a solid state).
Formation of ammonia in the presence of Fe(s), as a catalyst.
N2(g) +3H2(g) 2NH3(g)
In the above reaction, the phase of reactants is not the same as the phase of the catalyst used in the reaction. (i.e. reactants are in the gaseous state and catalyst is in the solid state).
NO formation in the presence of Pt(s), as a catalyst. 4NH3(g) + O2(g) 4NO(g) +6H2O(l)
In the above reaction, the phase of reactants is not same as the phase of the catalyst used in the reaction. (i.e. reactants are in the gaseous state and catalyst is in the solid state).
- On the basis of the effect on the chemical reaction, catalysts can be classified into different types:
Positive catalyst: It is the type of catalyst used in the chemical reaction which decreases the activation energy required to convert the conversion of product and thereby increasing the rate at which reaction takes place. When the catalyst is used it provides the alternate mechanism which is faster as compared to the mechanism in the absence of the catalyst. For example,
let us consider the reaction which takes place in the absence of a catalyst,
RP(uncatalysed reaction)
If the same reaction takes place in the presence of catalyst,
R+CatalystIntermediate
Intermediatecatalyst+P
The above reaction is known as a catalysed reaction in which the rate of catalysed reaction will be faster than the rate of the uncatalysed reaction.
Note: Although the catalyst participates in the reaction mechanism it is regenerated at the end of the reaction.
Inhibitor: It is also known as a negative catalyst used in the chemical reaction which decreases the rate at which reaction takes place by increasing the activation energy required to convert the conversion of product. When the negative catalyst is used, it either destroys the catalyst present in the system or may react with the reaction intermediate in a chain reaction thereby decreasing the rate of reaction.
Promoter: It is defined as the substance that itself does not act as a catalyst but increases the activity of the catalyst. Therefore the promoter when added to the reaction increases the activity of the catalyst without itself being involved in the chemical reaction. For example,
In the presence of Ni(s) as a catalyst and Cu as a promoter, vegetable oil is reduced to form vegetable ghee.
Vegetable oil(l) +H2 Vegetable ghee(s)
Fe(s) acts as a catalyst and Mo acts as a promoter in the formation of ammonia.
N2(g) +3H2(g) 2NH3(g)
Catalytic Poison: Substances that are not catalysts but whose presence reduces the activity of a catalyst. Poisoning is caused by the preferential adsorption of a chemical species (i.e. poison) on the catalyst's surface. These poisons can be present in the reaction in the form of impurity or may be formed as reaction by-products. In general, poison includes some compounds such as- H2S, CS2, PH3 etc or elements such as- As, Pb, Hg etc.
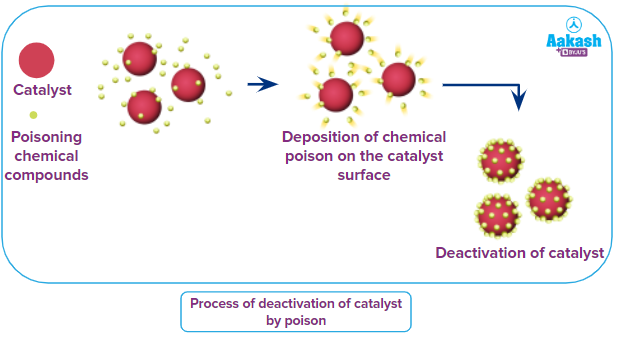
Auto catalyst: It is defined as the phenomena in which one of the products of a reaction acts as a catalyst for that reaction and thereby increases the speed of the chemical reaction.
RCOOR+ H3O+RCOOH+ROH
The above reaction is known as catalytic hydrolysis of the ester. When the concentration of reactant (H3O+) increases in the above reaction it results in an increase in the speed of the reaction. The concentration of (H3O+) increases due to the ionization of (RCOOH) formed in the product.
Effect of Catalyst on Rate of Reaction
Catalysts alter the reaction mechanism, thereby altering the rate of the reaction. A catalyst, in general, increases the rate of reaction by lowering the activation energy required for the reaction to proceed. In the case of inhibitors, which act as a negative catalysts, the rate of reaction slows as the activation energy rises due to the addition of negative inhibitors.
Let us consider the reaction which takes place in the absence of a catalyst,
RP(uncatalysed reaction)
If the same reaction takes place in the presence of catalyst,
R+CatalystIntermediate
Intermediatecatalyst+P
Here,
‘R’ is the reactant involved in the reaction
‘P’ is the product formed in the reaction
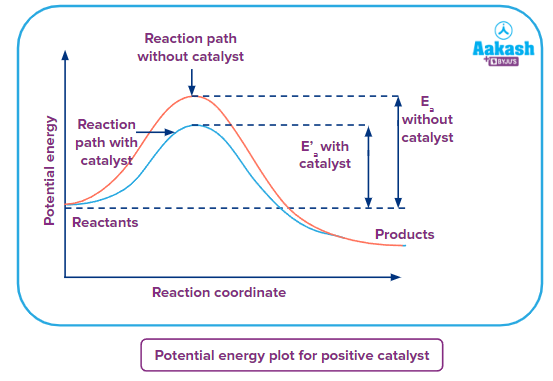
Potential energy vs Reaction Coordinate curve
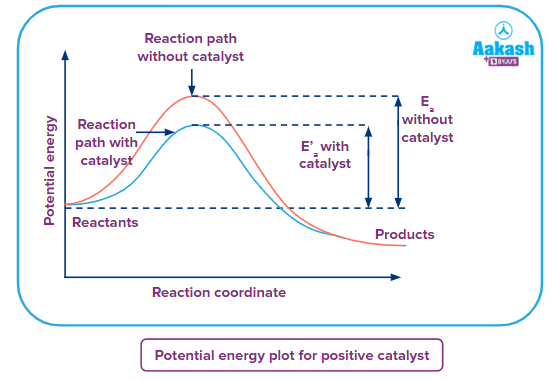
As from the above graph it can be seen that if the reaction takes place in the absence of a catalyst, it requires crossing a higher energy barrier known as activation energy represented by Ea . In the case of reaction taking place in the presence of the positive catalyst the energy barrier(activation energy) E'a , is lower than the activation energy of the uncatalysed reaction. Therefore, the rate of reaction in the presence of catalyst is more than the rate of the uncatalysed reaction.
Potential Energy vs Reaction Coordinate Curve
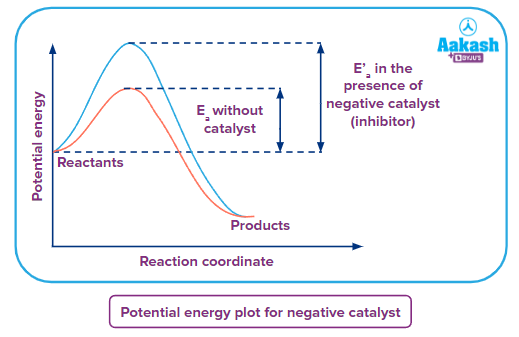
From the above graph, it can be seen that the reaction that takes place in the absence of a catalyst requires crossing the lower energy barrier known as activation energy represented by Ea in the above graph whereas, in the case of reaction taking place in the presence of the negative catalyst or inhibitor, the energy barrier(activation energy) E'a is higher than the activation energy of the uncatalysed reaction. Therefore, the rate of reaction in the presence of catalyst is less than the rate of the uncatalysed reaction.
Practice Problems
Q1. Which of the following option is correct for a catalytic reaction?
- In the presence of the catalyst reaction rate remain unchanged
- In the presence of the catalyst the rate of reaction may increase or decrease depending on the type of catalyst used.
- Rate of reaction always increases in the presence of catalyst
- Catalyst is completely consumed when it is involved in a chemical reaction.
Answer: (B)
Solution: A catalyst's mass and chemical composition remain unchanged during the reaction. Its physical state, on the other hand, is subject to change. Catalysts are defined in chemistry as substances that change the rate of a reaction by changing its path. A catalyst is used to both accelerate or decrease the rate of a reaction depending upon the catalyst used in the chemical reaction. For example, Phosphoric acid acts as a negative catalyst, slowing the decomposition of hydrogen peroxide. When potassium chlorate is heated to 700 degrees Celsius, it decomposes to produce oxygen gas. When MnO: is added, the decomposition occurs at 300 degrees Celsius. Therefore, Option (B) is correct.
Q2. The chemical reaction in which the physical state of reactant and catalyst is different is known as _________.
a. Auto catalysis
b. Catalytic Poison
c. Homogeneous catalyst
d. Heterogeneous catalyst
Answer: (D)
Solution: Heterogeneous catalyst is defined as the reaction in which the phase of the catalyst and the reactants used in the reaction is different. For example let us consider a reaction,
Decomposition of H2O2 in presence of MnO2(s), as a catalyst.
2H2O2(l) H2O(l) +O2(g)
In the above reaction, phase of reactant is not same as the phase of the catalyst used in the reaction. (i.e. reactant is in liquid state and catalyst is in solid state). Therefore, option (D) is correct.
Q3. Select the correct option for the relationship between activation energy and the positive catalyst used in the chemical reaction.
a. In the presence of positive catalyst activation energy is lower than the activation energy of the uncatalysed reaction.
b. In the presence of positive catalyst activation energy is equal to the activation energy of the uncatalysed reaction.
c. In the presence of positive catalyst activation energy is higher than the activation energy of the uncatalysed reaction.
d. Can’t be determined
Answer: (A)
Solution: Catalysts alter the reaction mechanism, thereby altering the rate of the reaction. A catalyst, in general, increases the rate of reaction by lowering the activation energy required for the reaction to proceed.
In the graph given below, it can be seen that if the reaction takes place in the absence of a catalyst it requires to cross the higher energy barrier known as activation energy represented by Ea in the above graph whereas in the case of reaction taking place in the presence of the positive catalyst the energy barrier(activation energy) is lower than the activation energy of the uncatalysed reaction.
Q4. Which among the given option does not get affected/changed by the addition of catalyst?
a. The rate of reaction
b. Activation energy of the reaction
c. Mechanism of the reaction
d. Spontaneity of the reaction
Answer: (D)
Solution: When the catalyst is added to a chemical reaction, it changes the rate of reaction, mechanism of the reaction, the activation energy of the reaction but does not change spontaneity of the reaction. Therefore, option (D) is correct.
Frequently Asked Question-FAQs
Q1. What are the different factors that affect reaction rate?
Answer: There are different factors that affect reaction rate which include:
- Nature of reactants
- Concentration of reactants
- Physical state of reactants
- Solvent
- Surface area of reactant
- Catalyst
- Temperature
Q2. What is the effect of the catalyst on the equilibrium of the reaction?
Answer: In the case of a reversible reaction when the catalyst is added the rate of both forward reactions as well the rate of backward reaction changes. But the change in the rate of forwarding reaction and backward will be the same and therefore it does not change the equilibrium state. Rather the equilibrium state is established faster.
Q3. What is the application of catalysts in everyday life?
Answer: An enzyme is a biological catalyst. Enzymes are required to control cell reactions. They are also important in the industry. In chemistry, a catalyst is any material that increases the rate of a reaction without itself participating in the reaction. Enzymes are naturally occurring catalysts that are responsible for many basic biochemical reactions.
However, apart from aluminium oxide and silicon dioxide, other oxides are important catalysts. In the Contact Process, for example, vanadium(V) oxide on the silica surface is the catalyst for the oxidation of sulphur dioxide to sulphur trioxide: potassium sulphate is added as a promoter.
Q4. What is activation energy?
Answer: When there is insufficient energy, reactions stop, transition states do not form, and products do not form. The minimum amount of energy required by the reactant molecules to react and form a product is known as activation energy.


