-
Call Now
1800-102-2727
Dinitrogen- Introduction, Preparation, Physical and Chemical properties, Anomalous Behaviour of Nitrogen & Uses
Have you seen tyres getting filled with nitrogen gas instead of compressed air? Yeah, that's true, there are stations where you can get your bike and car tyres filled with nitrogen gas. This is a pretty new technology and it’s quite popular these days. But do you know the reason behind it, why air is replaced with nitrogen gas when it comes to the inflation of tyres? We all know that at high temperatures, the pressure of the gas increases considering the fixed amount of gas in a confined space. So, nitrogen gas works perfectly fine under those conditions, nitrogen gas provides a stable pressure range with respect to temperature.
Moreover, nitrogen gas is chemically inert in nature; it does not react with the inside rubber layer of the tyre, on the other hand, oxygen in the air oxidises the rubber material and can hamper the tyre at much faster rate. Nitrogen was discovered in 1772 by the Scottish chemist Daniel Rutherford. The name nitrogen is derived from the word nitre a well-known nitrogen compound. Let’s understand more about the properties and uses of dinitrogen or nitrogen gas.


Table of Contents
- Introduction to Dinitrogen
- Preparation of Dinitrogen
- Physical Properties of Dinitrogen
- Chemical properties of Dinitrogen
- Anomalous Behaviour of Nitrogen
- Uses of Dinitrogen
- Practice Problems
- Frequently Asked Questions - FAQs
Introduction to Dinitrogen
Nitrogen is widely distributed in the air both in free as well as in combined form. Air is the most abundant source of nitrogen. It forms 75% by mass and 78% by volume of air. In combined form it is found as nitrate such as Chile saltpetre (NaNO3), Indian saltpetre (KNO3) and ammonium compounds. Nitrogen is an essential constituent of all living organisms, found in the form of protein, amino acid and nucleic acid. Nitrogen exists in elemental form as a diatomic molecule (N2) and it is therefore called dinitrogen. Dinitrogen is a linear molecule with sp hybridisation that contains one 𝜎 bond and two 𝜋 bonds.
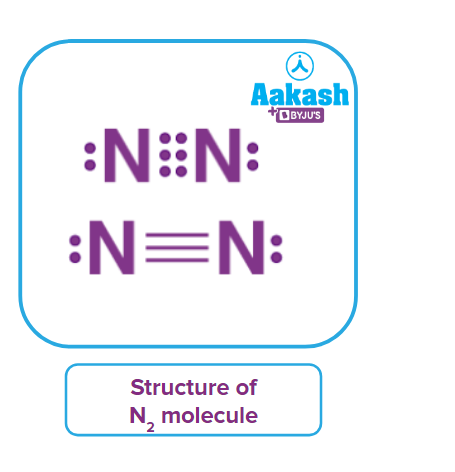
Preparation of dinitrogen
Dinitrogen is conveniently prepared in the laboratory by the following methods:
- Heating of ammonium nitrite
Ammonium nitrites on heating liberate nitrogen gas.
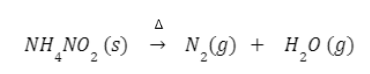
The above reaction is explosive therefore the equivalent amount of ammonium chloride and sodium nitrite is warmed to form nitrogen gas.
- Oxidation of ammonia
Dinitrogen can be prepared from the oxidation of ammonia with Cl2, bleaching powder, sodium hypochlorite, copper oxide and lead oxide.
- Preparing Dinitrogen by heating urea from an acidified solution of nitrite
When urea is heated with an acidified solution of nitrite (i.e., nitrous acid), it produces nitrogen gas along with carbon dioxide and water.
![]()
- Heating of ammonium dichromate
When ammonium dichromate (orange-red in colour) is heated, a violent reaction takes place which is accompanied by flashes of light and nitrogen gas is emitted leaving behind a green colour residue of chromic oxide (Cr2O3).
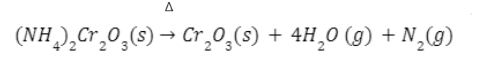
- By heating of barium azide/sodium azide
Very pure nitrogen gas is obtained by heating of barium azide or sodium azide.
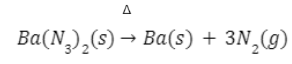
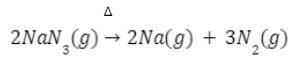
- Commercial Preparation of Dinitrogen
Nitrogen gas is present in the air in the form of a mixture along with other gases like carbon dioxide, oxygen, water vapour, and inert gases. Nitrogen gas is manufactured by fractional distillation of liquid air. The boiling point of liquid nitrogen is -196 ℃ or 77.3 K. There is a sufficient difference in the boiling point of liquid air and liquid nitrogen which can be separated by Claude’s process.
Physical properties of dinitrogen
- Nitrogen is a colourless, odourless and tasteless gas.
- It is sparingly soluble in water (2.3 volumes in 100 volumes at normal temperature and pressure).
- It has a melting point of 62.5 K and boiling point of liquid nitrogen is -196 ℃ or 77.3 K.
- It is slightly lighter than air and non-poisonous.
- It is neither combustible nor a supporter of combustion.
Chemical properties of dinitrogen
Dinitrogen is an inert diatomic gas containing (one 𝜎 bond and two 𝜋 bonds). It has an interatomic distance of 109.5 pm. It has a high bond dissociation energy and bond order of 3.0. It dissociates into atoms at very high temperature and hence it is chemically less reactive in nature.
Important chemical reactions of nitrogen are as follows:
- Nitrogen gas combines with the electropositive metals to form corresponding metals nitrides. For example,
- Nitrogen gas reacts with calcium carbide forming calcium cyanamide.
- Nitrogen gas reacts with oxygen only at a high temperature approximately 3273 K to form nitric oxide and the reaction is reversible in nature.
N2(g)+O2(g) ⇌ 2NO(g)
- Nitrogen combines with hydrogen gas at high temperatures (673 K to 823 K ) and high pressure of (100-1000 atm) to produce ammonia. This reaction of formation of ammonia is also the commercial method of preparation of ammonia on large scale and is known as Haber's process.
N2(g)+3H2(g) ⇌ 2NH3(g)
Anomalous behaviour of nitrogen
Nitrogen's abnormal behaviour is due to its small size, high ionisation energy, high electronegativity, and non-availability of valence electrons in the d-orbitals.
A few reasons for the anomalous behaviour of nitrogen are as follows:
- Small size: Nitrogen has a high electronegativity value, a high ionisation enthalpy, and no vacant d-orbitals due to its small size.
- 𝜋-bond formation: N2 is exceptional in its capacity to form p-p multiple bonds as the effective overlapping is quite possible when two atoms of nitrogen come closer to form a bond due to its small size, but heavier members of group-15 (i.e., on moving down the group) elements are unable to make p-p multiple bonds because their atomic orbitals are too vast and diffused to allow effective overlapping.
- Nitrogen gas is a diatomic molecule having a triple bond between the two atoms, whereas other elements in their elemental state can only form single bonds.
Uses of Dinitrogen
Dinitrogen is used for different purposes which include:
- Nitrogen is a relatively unreactive element because it forms a stable triple bond, therefore it is used to provide inert atmosphere in certain metallurgical operations and during welding as well.
- Large quantity of nitrogen is used as a blanket air to protect the material from oxygen during processing or storage.
- It is used in the manufacturing of electronic components.
- It is used as a raw material in manufacturing NH3, HNO3, CaCN2 and other nitrogen containing compounds.
- Liquid nitrogen is used as a refrigerant to freeze food and for the preservation of food during transportation as the boiling point of liquid nitrogen is -196 ℃ or 77.3 K.
- Liquid nitrogen is also used in cryopreservation technique to preserve the tissue, embryo as its boiling point of liquid nitrogen is -196 ℃ or 77.3 K.
Practice problem
Q. Which of the following compounds on heating does not produce nitrogen gas?
a) Ammonium nitrite
b) Ammonium nitrate
c) Ammonium dichromate
d) Barium azide
Answer: (B)
Solution:
When ammonium nitrate is heated nitrous oxide (N2O) is formed along with water molecules (H2O).
NH4N03N2O+2H2O
Remaining all on heating produces nitrogen gas.
Ammonium nitrites on heating liberate nitrogen.
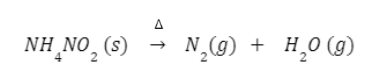
When ammonium dichromate (orange-red in colour) is heated, a violent reaction takes place which is accompanied by flashes of light and nitrogen gas is emitted leaving behind a green colour residue of chromic oxide (Cr2O3).
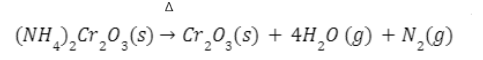
Very pure nitrogen gas is obtained by heating of barium azide.
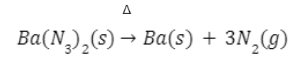
Q. Which gas will be released when ammonia is passed through the heated CuO?
- NO2
- O2
- N2
- N2O
Answer: (C)
Solution:
Nitrogen gas is released when ammonia gas is passed through the heated copper oxide (CuO).
Q. Which of the following explains the reason for chemically inertness of nitrogen gas at a lower temperature?
a) Stable electronic configuration
b) Small bond dissociation energy
c) High bond dissociation energy
d) Lower electronegativity
Answer: (C)
Solution: Dinitrogen is an inert diatomic gas containing(one 𝜎 bond and two 𝜋 bonds). It has an interatomic distance of 109.5 pm. it has a high bond dissociation energy of 3.0. It dissociates into atoms at very high temperatures and hence it is chemically inert in nature.
Q. What will be the nature of N2 the molecule in terms of its magnetic behaviour? Explain it using the molecular orbital theory.
Answer: In the case of nitrogen molecule the electrons are filled in both the bonding and antibonding molecular orbitals.
The molecular electronic configuration of N2 is as follows:
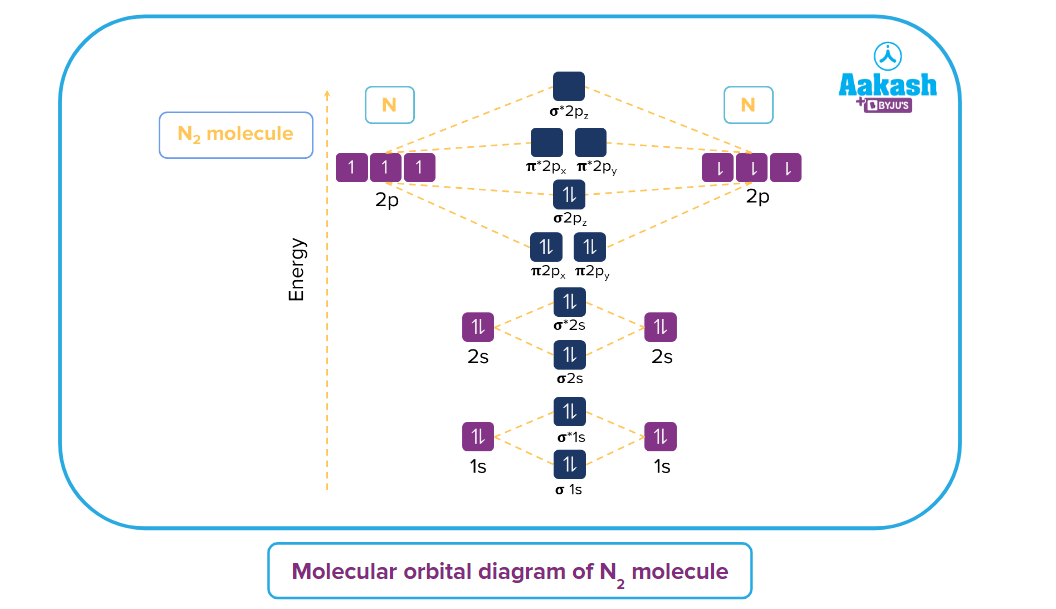
As you can see, all the electrons are paired up. So, this molecule is diamagnetic in nature.
Frequently asked question-FAQs
Q. What will happen if we breathe pure nitrogen gas?
Answer: As we know that nitrogen is an inert gas, which means it doesn't react chemically with other gases and isn't poisonous. However, breathing pure nitrogen is lethal. This is due to the gas's ability to displace oxygen in the lungs. Within one or two breaths, you may go unconscious.
Q. How liquid nitrogen helps in the preservation of food during transportation?
Answer: When the food is transported for a longer distance, the vehicle generally has a setup to carry the liquid nitrogen cylinders which help in maintaining the temperature of the chamber in which food is kept the boiling point of liquid nitrogen is -196 ℃ or 77.3 K . It takes away the heat as soon as it is sprayed and decreases the temperature of the food sample and at a lower temperature, the metabolic and enzymatic reaction also seizes and thereby preserving food from spoilage.
Q. How does the formation of nitrogen molecules take place?
Answer: As we know that the atomic number of nitrogen is 7 with an electronic configuration 1s22s22p3 It requires 3 more electrons to complete its octet and therefore it shares three electrons with other nitrogen atom forming a triple bond.
Q. What is the importance of nitrogen in plant growth?
Answer: Nitrogen is a significant component of amino acids, which are the building blocks of plant proteins and enzymes, and is an essential macronutrient for plant activity. Proteins are the structural ingredients of all living things, and enzymes help plants perform a wide range of biochemical activities. Nitrogen is also included in the chlorophyll molecule, which allows the plant to capture sunlight energy through photosynthesis, resulting in increased plant growth and grain output.


