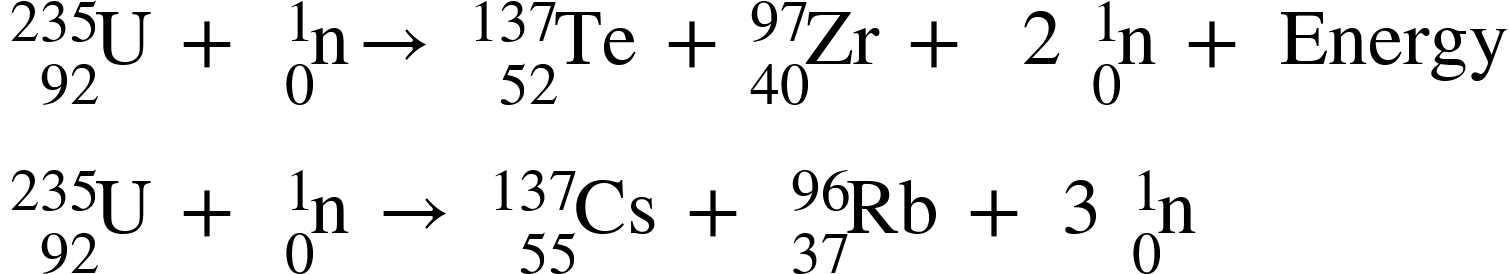-
Call Now
1800-102-2727
Difference between Fission and Fusion- Introduction to Nuclear Fission and Fusion Reactions, Practice Problems and FAQs
When India was fighting for its independence, the rest of the world was fighting for its ideology and dominance. But do you know what happened in the cities of Japan during world war-II?
Two atomic bombs were dropped in the cities of Hiroshima and Nagasaki and the evidence of the same still persists. Have you ever wondered, what fuel the atomic bombs which can cause destruction at this level? What kind of reactions occurs when an atomic bomb explodes?
In atomic bombs, uncontrolled nuclear reactions happen. But do you know what kind of nuclear reactions occurs in the atomic bomb?

In this article, we will learn about the two most important types of nuclear reactions and will be discussing the differences between these two nuclear reactions.
Table of Contents
- Introduction to Nuclear Fission Reaction
- Introduction to Nuclear Fusion Reaction
- Difference between Nuclear Fission and Nuclear Fusion Reaction
- Practice Problems
- Frequently Asked Questions - FAQs
Introduction to Nuclear Fission Reaction
Two German scientists, Fritz Strassmann and Otto Hahn, discovered the nuclear fission reaction in 1938. Nuclear fission occurs when the nucleus of a heavier radioactive atom splits into lighter nuclei as a result of a nuclear reaction. This decay can happen naturally as a result of radioactive decay, or it can be simulated by creating the right conditions (bombarding with neutrinos). The resulting fragments have a lower combined mass than the original. In the preceding reaction, the missing mass is converted into nuclear energy. As a result, nuclear fission is defined as the process by which an atom's nucleus splits into two or more daughter nuclei.
Introduction to Nuclear Fusion Reaction
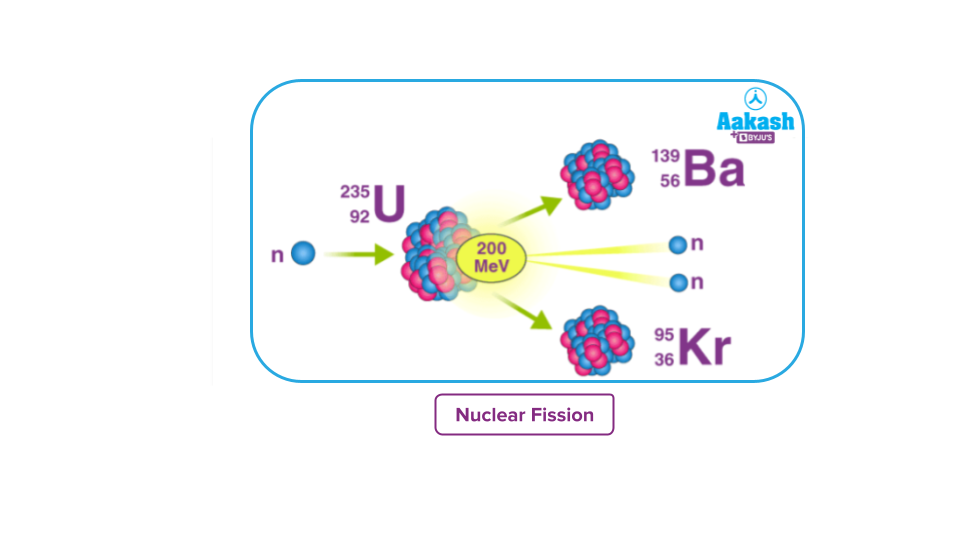
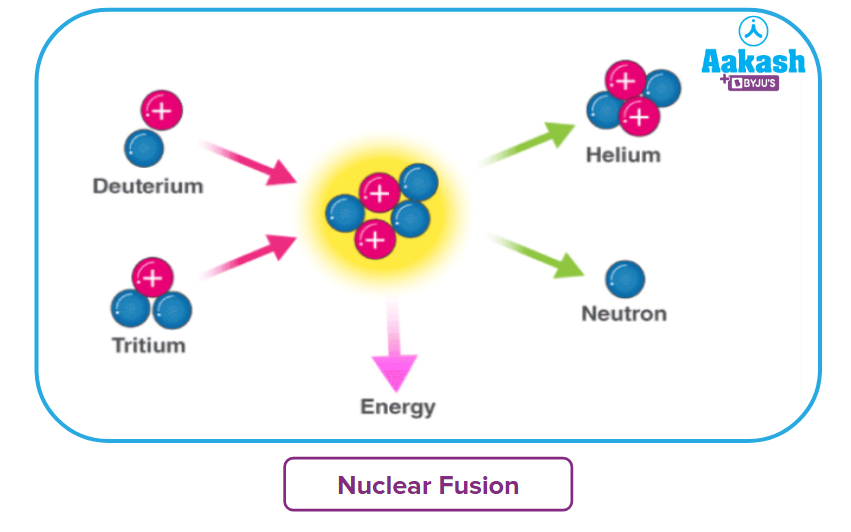
When two or more lighter nuclei combine to form heavier nuclei, a nuclear fusion reaction occurs. Subatomic particles such as protons and neutrons are also produced as byproducts. This type of reaction is also known as a thermonuclear reaction.
Consider the fusion of deuterium and tritium to produce helium and neutrons, which release huge amounts of energy and are exothermic in nature because heavier nuclei are formed from two lighter nuclei. The participating nuclei must be brought together for the nuclear fusion reaction to occur. They should be brought close enough together that the nuclear forces activate and bind the nuclei together.
Difference between Nuclear Fission and Nuclear Fusion Reaction
|
Nuclear fission |
Nuclear fusion |
|
Heavier nuclei break into lighter or smaller nuclei in this type of reaction. |
In this reaction, lighter nuclei combine together to form heavier nuclei. |
|
In this reaction, there is a chain reaction that occurs. |
In this type of reaction generally, a chain reaction takes place. |
|
It does not necessarily require a high temperature. |
Fusion requires an extremely high temperature to occur. |
|
Nuclear waste is produced at the end of the fission reaction. |
No nuclear waste is produced at the end of the fusion reaction. |
|
The products obtained in the reaction are radioactive in nature. |
The products obtained from the fusion reaction are non-reactive in nature. |
|
For example: |
For example: |
Practice Problems
Q1. Select the correct option to classify the given nuclear reaction?
![]()
- Nuclear fusion reaction
- Nuclear fission reaction
- Projectile-capture reaction
- Both A and B
Answer: (A)
Solution: Nuclear fission occurs when the nucleus of an atom of heavier radioactive nuclei splits into lighter nuclei as a result of a nuclear reaction. For example,
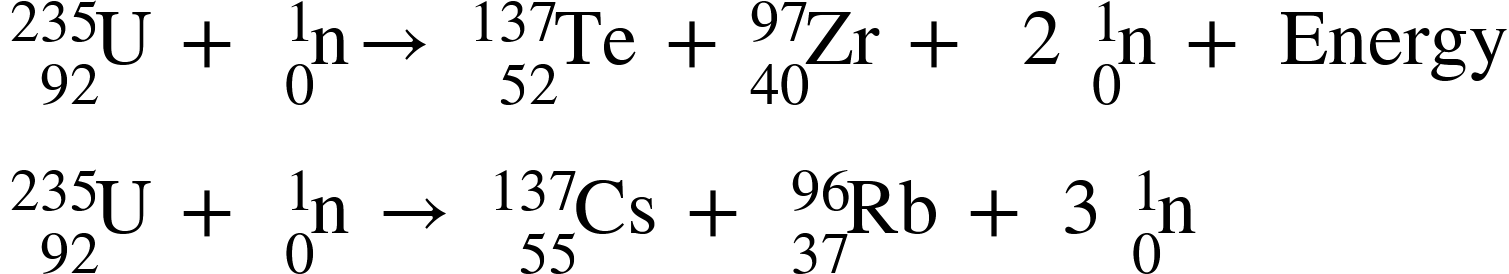
The projectile capture reaction results in the formation of an unstable nucleus which achieves stability through the emission of nuclear particles. The particles which are bombarding usually get absorbed by ray emission.
Let's consider an example,
![]()
When two light nuclei combine to form heavier nuclei, a nuclear fusion reaction occurs. Subatomic particles such as protons and neutrons are also produced as byproducts.
![]()
Therefore option (A) is the correct answer.
Q2. Select the correct option with respect to a nuclear fission reaction.
- This reaction takes place inside the core of the sun.
- It is endothermic in nature as the mass loss converts into energy.
- It occurs when two light nuclei combine to form heavier nuclei.
- The nuclear waste is produced in the nuclear fission reaction.
Answer: (D)
Solution: Nuclear fusion reaction takes place inside the core of the sun and is exothermic in nature in which two lighter isotopes of hydrogen combine in the core of the sun at high temperature and pressure to form helium nuclei.
![]()
In the case of nuclear fission reaction, heavier nuclei dissociate into two or more lighter nuclei which is generally unstable and radioactive in nature.
Therefore, option (D) is the correct answer.
Q3. Select the correct option for the type of reaction that occurs when unstable heavier nuclei split into lighter nuclei during radioactive decay.
- Nuclear fission
- Nuclear fusion
- Thermonuclear reaction
- Both B and C
Answer: (A)
Solution: Nuclear fission occurs when the nucleus of an atom of heavier radioactive nuclei splits into lighter nuclei as a result of a nuclear reaction. For example,
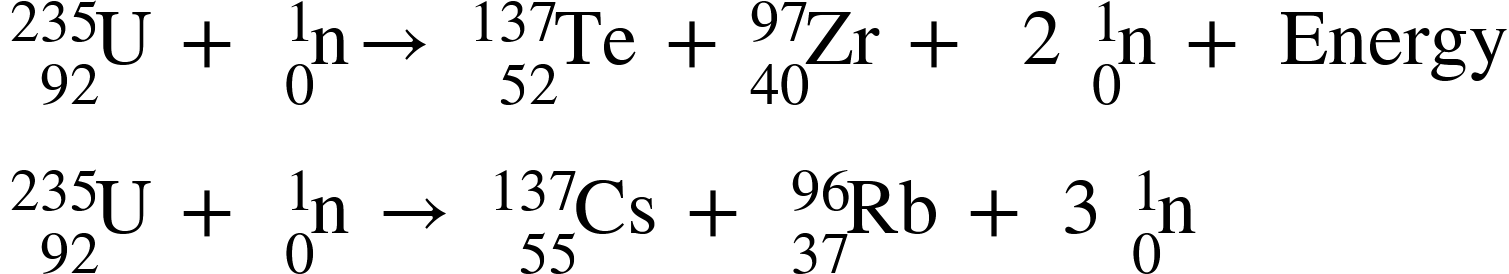
In a nuclear fusion reaction, two lighter nuclei combine to form heavier nuclei. Subatomic particles such as protons and neutrons are also produced as byproducts. This type of reaction is also known as the thermonuclear reaction.
![]()
Therefore, option (A) is the correct answer.
Frequently Asked Questions - FAQs
Q1. List some important applications of the nuclear fission reaction.
Answer: Nuclear fission reaction has a wide range of applications which includes:
- It is used in the working of an atom bomb.
- Generating electricity in nuclear power plants.
- It is used to produce some lighter nuclei like Cs-137, Te-137 etc.
- It is used as a source of propellant in submarines.
Q. What is a nuclear reactor?
Answer: The reactor is a furnace that burns fissionable materials for a useful purpose. It is the instrument designed to control the development of a nuclear chain by not allowing all neutrons produced to carry the chain reaction. Controlled fission is carried out in a nuclear power plant.
Q2. How is the nuclear fission reaction controlled in a nuclear power plant?
Answer: It is essential to control the chain reaction which occurs during the nuclear fission reaction such that the energy in the reaction is produced at a usable rate. Controlled rods made up of cadmium steel or boron rods are used to absorb the neutrons produced by uranium-235 fission and thus control the rate of the fission reaction.
![]()
Q3. How to determine the nuclear stability of an element?
Answer: The natural tendency of an atom nucleus to decay or change into a stable element is referred to as nuclear stability. If an element's isotope (referred to as a nuclide) is unstable, the nuclide has an inclination to emit radiation and is referred to as an unstable radioactive nuclide. The neutron-proton ratio (n/p) is useful in determining nuclear stability. This ratio is close to one for atoms with low atomic numbers (less than about 20 protons). The n/p ratio steadily rises as the atomic number increases past the elements with atomic number 20.
Q4. What is a nuclear chain reaction?
Answer: A nuclear chain reaction is a process in which neutrons released during fission cause another fission which produces at least one additional nucleus. This nucleus then emits neutrons, and the cycle continues. The process can be either controlled (using nuclear power) or uncontrolled (nuclear weapons).