-
Call Now
1800-102-2727
Diels-Alder Reaction–Definition, Mechanism, Stereochemistry, Variations, Applications, Practice Problems & FAQs
Miracles do happen! But there is a hidden mystery behind every miracle that we observe. A majority of scientists believe in this! This is what keeps the fire of invention alive in them.
In current times, efforts of multiple scientists have bore sweet fruits as they could actually transform several substances through a series of chemical reactions to synthesise new chemical compounds. And this method of producing synthetic organic compounds has transformed lives of the commoners and influenced the development of the world like no other.
That is in fact no less than magic! Diels-Alder reaction is one such typical organic transformational reaction, and the final product obtained is indeed magical!
Let’s think of an imaginary situation! One fine evening, four brothers Rafael, Usain, Roger and Lionel were playing passing the ball in a garden. Soon enough, Cristiano and David came there and expressed their wish to play with them. They were lovingly accepted by the four siblings playing with the ball. And they asked the duo to join hands with them such that they stand in the form of a hexagonal ring.
With all the love, the siblings extended their hands and Cristiano and David also reverted the same gesture. Now they stood as is revealed in this image!
Diels Alder reaction too is quite similar to how this 4-brother+2-brother addition took place!
The group of four who were already playing, warmly accepted the duo brothers and together they formed a hexagonal circle!
An American organic chemist and Nobel laureate of 1990, Elias James Corey made use of the Diels-Alder reaction in the early stages of synthesis of prostaglandins. Synthetic prostaglandins are most frequently used to cure glaucoma, although they can also be used to treat stomach ulcers, induce labour, treat erectile dysfunction, and treat pulmonary hypertension.
To quote him, “The Diels-Alder is one of the most important and fascinating transformations in chemistry and continues to surprise, excite, delite and inform the chemical community.”
Table of Contents
- Diels-Alder Reaction-Definition
- Mechanism of Diels-Alder Reaction
- Variation of Diels-Alder Reaction
- Regioselectivity in Diels-Alder Reaction
- Stereochemistry of Diels-Alder
- Characteristics of Diels-Alder Reaction
- Applications of Diels-Alder Reaction
- Practice Problems
- Frequently Asked Questions-FAQs
Diels-Alder Reaction–Definition
A substituted alkene (dienophile) and a conjugated diene are reactants in the significant organic chemical process known as the Diels-Alder reaction. The term "dienophile" is frequently used to describe this substituted alkene. A cyclohexene substituted derivative results from this reaction. A particularly good illustration of pericyclic reactions that take place through coordinated processes is the Diels-Alder reaction (i.e., all bond breakage and bond formation occurs in a single step).
The German chemists Otto Diels and Kurt Alder discovered this reaction and developed 1,4-cycloaddition in 1928, for which they were awarded the Nobel Prize in 1950 in Chemistry. Due to the simultaneous formation of two additional carbon-carbon bonds, the Diels-Alder process can create six-membered rings. The simplest of Diels-Alder reactions that takes place would be in between 1,3-butadiene (diene) and ethene (dienophile).
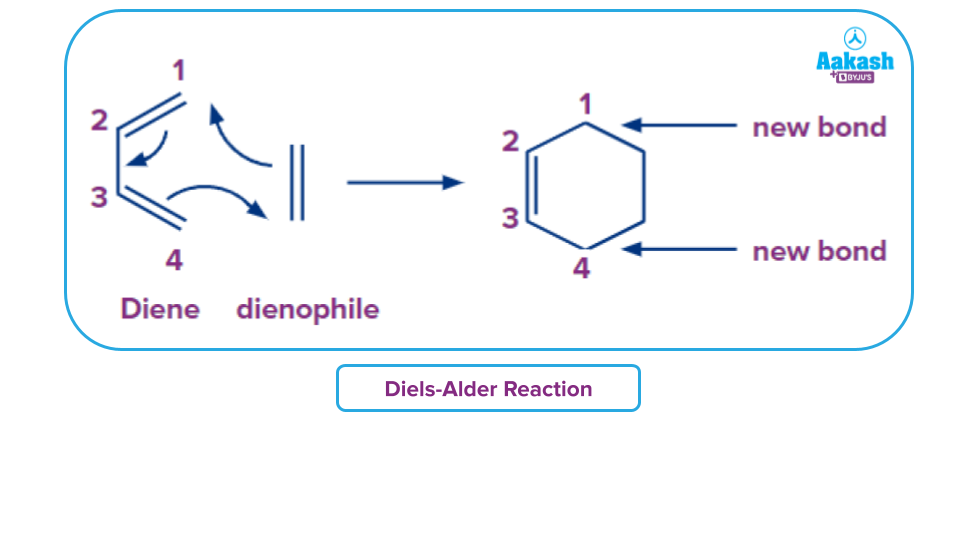
The synthesis of vitamin B6 uses this process. On a large scale, cyclopentadiene is created using the reverse reaction, also known as the retro-Diels-Alder process.
Mechanism of Diels-Alder Reaction
Diels-Alder reaction is a pericyclic reaction with a concerted mechanism. Electrophilic dienophiles with electron-withdrawing groups like -CHO, >C=O, -COOR, -NO2, -CN linked to them are favourable for the Diels-Alder process. It is also favoured by electron-donating groups in nucleophilic dienes. A few excellent dienes and dienophiles for the Diels-Alder reaction examples are provided below.
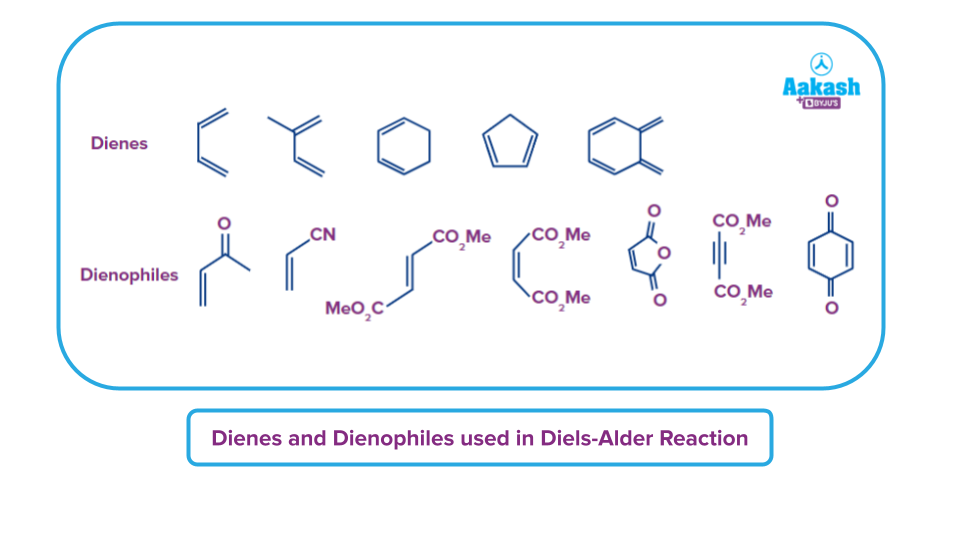
The process is a one-step cycloaddition reaction because the Diels-Alder reaction mechanism is concerted. Here, a cyclic adduct is created when two unsaturated molecules come together. Bond multiplicity has decreased overall. Bond creation and bond destruction all take place at once. An example of the single step concerted mechanism is provided below:
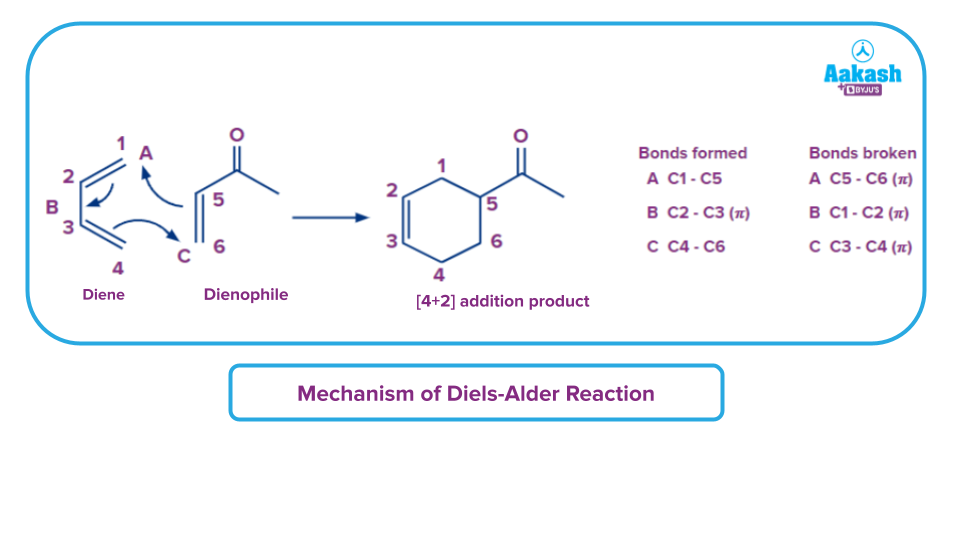
The result of this reaction between the diene and dienophile is a derivative of cyclohexene. The mechanism's image shows that just one 𝜋 bond forms in place of the three carbon-carbon 𝜋 bonds that break and two sigma bonds are created.
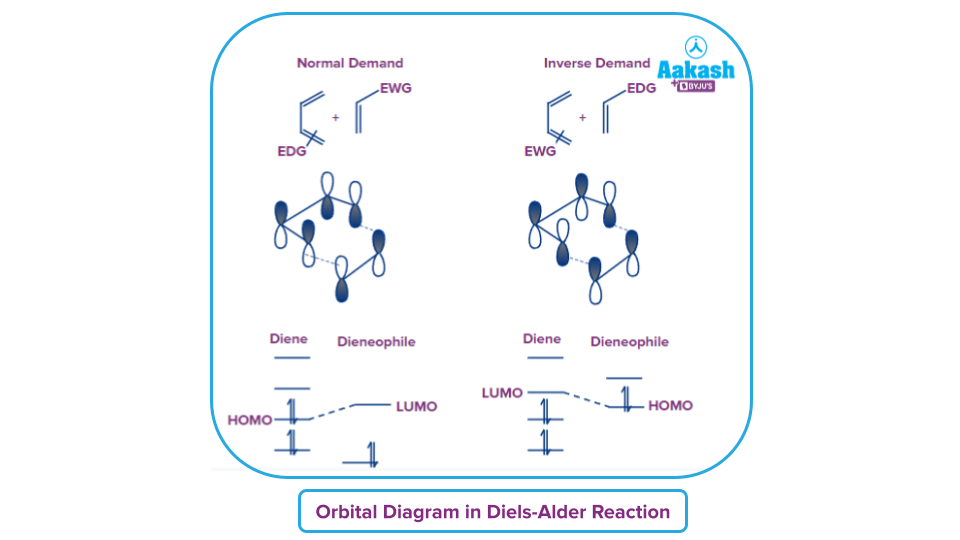
Variation of Diels-Alder Reaction
Variations of Diels-Alder Reaction are:
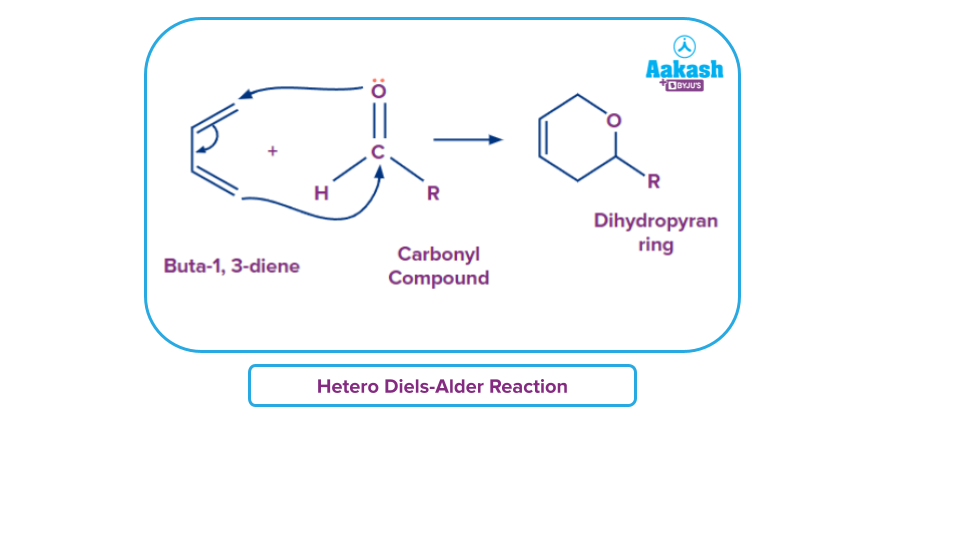
Hetero-Diels Alder
- These reactions involve one or more heteroatoms (any atom other than carbon or hydrogen).
- Dihydropyran products are created when dienes and carbonyl groups react.
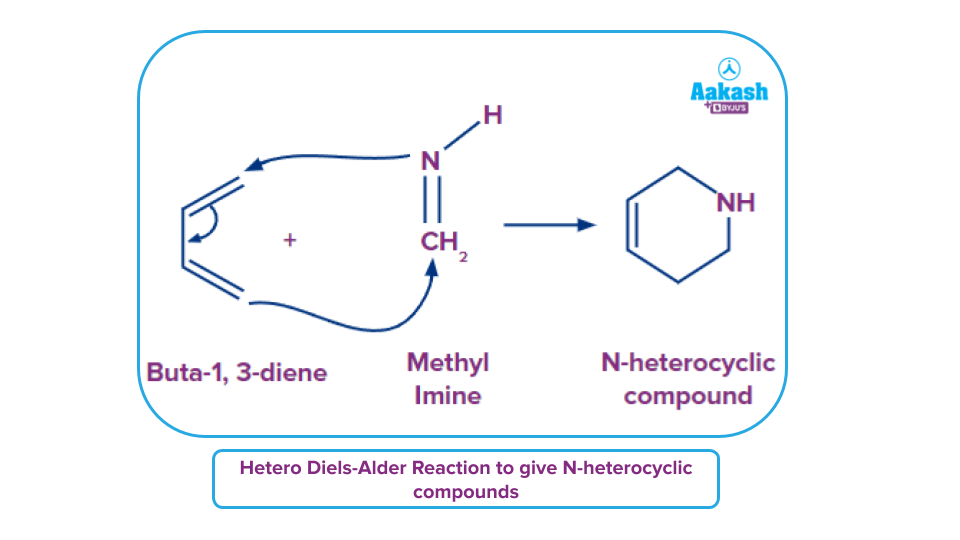
- The dienophile in the Diels-Alder reaction is an imine (or diene substituents). N-heterocyclic molecule is the end product of this reaction.
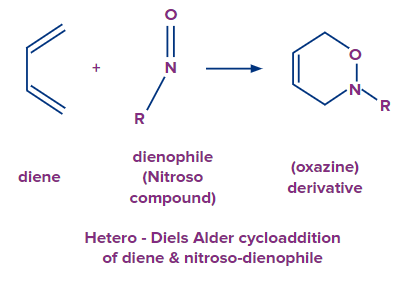
- When a nitroso (R-N=O) molecule is utilised as the dienophile, they react with dienes to produce oxazines.
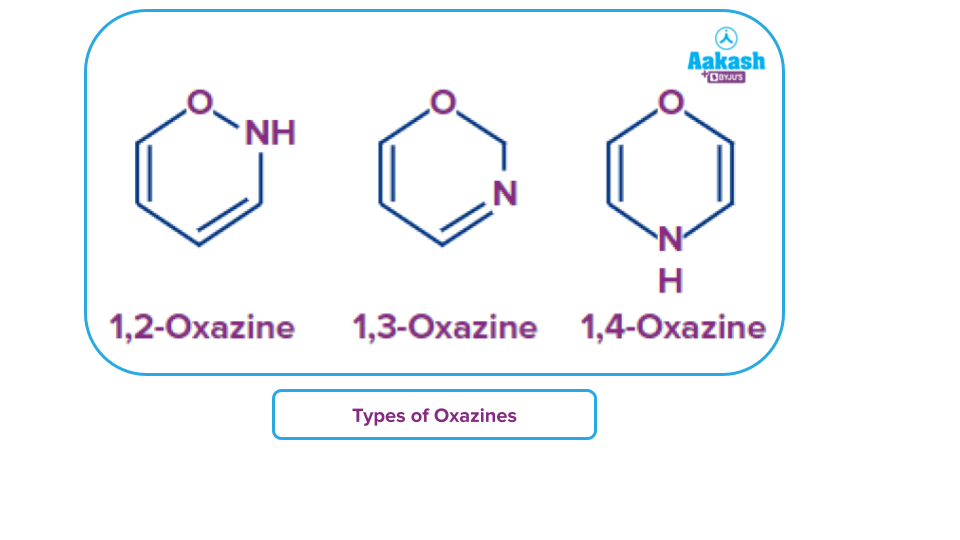
Lewis Acid Application
- In this version, the catalyst is a Lewis acid..
- Lewis acids like aluminium chloride, boron trifluoride, tin tetrachloride, and zinc chloride can be used in these reactions.
- The dienophilicity of the dienophile complex is increased in these reactions by the Lewis acid.
- Benefits of this variety include faster response times and improved stereo- and regioselectivity. It is possible for these Diels-Alder reactions to happen at low temperatures.
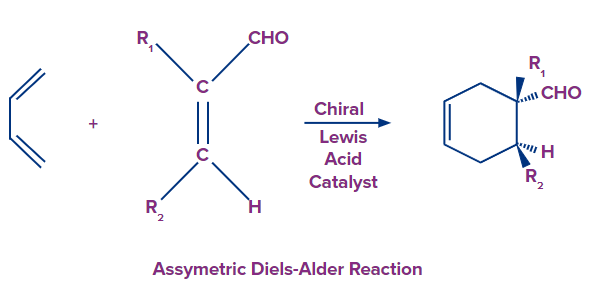
Asymmetric Diels Alder Reaction
- Asymmetric Diels Alder reactions are important in producing optically active six-membered heterocyclic rings with high stereoselectivity.
- This method uses chiral Lewis acids or chiral boron complexes as catalysts to produce enantiomers.
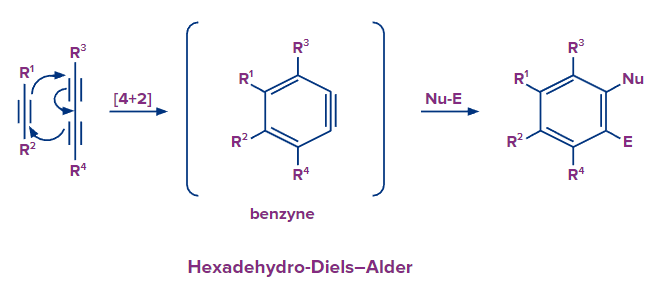
Hexadehydro Diels-Alder Reaction
- Alkynes and diynes, as opposed to alkenes and dienes, are utilised in the hexadehydro Diels-Alder reaction to create an unstable benzyne intermediate that may then be trapped to create an aromatic product.
- This reaction enables the rapid synthesis of highly functionalized aromatic rings.
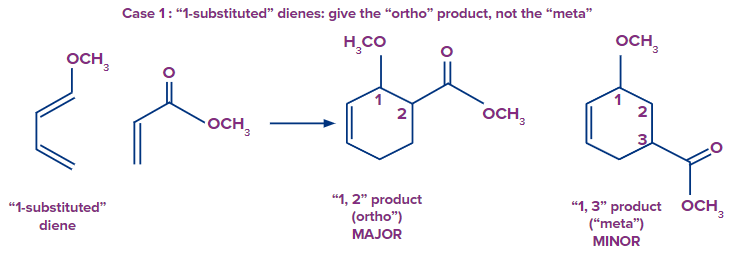
Regioselectivity in Diels-Alder Reaction
The preference for chemical bond formation or breaking of bond in one direction over all other potential ways is known as regioselectivity in chemistry. For example- Hydrohalogenation of alkenes following Markonikov’s rule is an example of regioselectivity.
The electron distribution in the diene and the dienophile is what causes regioselectivity. The diene's most electron-rich carbon interacts with the dienophile's most electron-deficient carbon. Consider the diene to be a nucleophile and the dienophile to be the electrophile. Regioselectivity arises from the combination of the extreme nucleophilic part of diene with the electrophilic part of dienophile.
The ortho-para rule can be used to predict the regioselectivity of the Diels-Alder reaction between unsymmetrical dienes and unsymmetrical dienophiles. Two regioisomers are feasible when unsymmetrical dienes and unsymmetrical dienophiles interact. Ortho refers to the tendency of dienes with substituents at the terminal to produce "1,2" products. Dienes that have a substituent at 2nd position, ("2-substituted dienes") frequently yield the "1,4" product ("para"). "1,3" products ("meta") are often just minor by-products. The Diels-Alder favours a one kind of connection, in order words, it is regioselective.
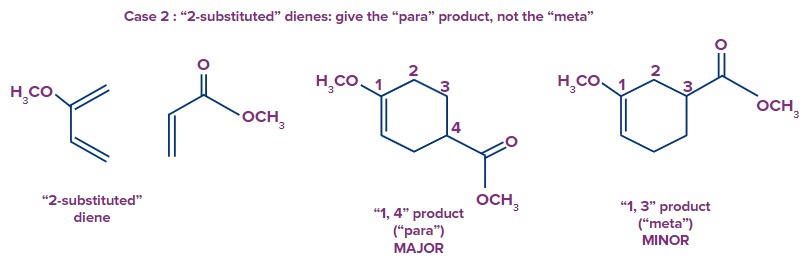
Stereochemistry in Diels-Alder Reaction
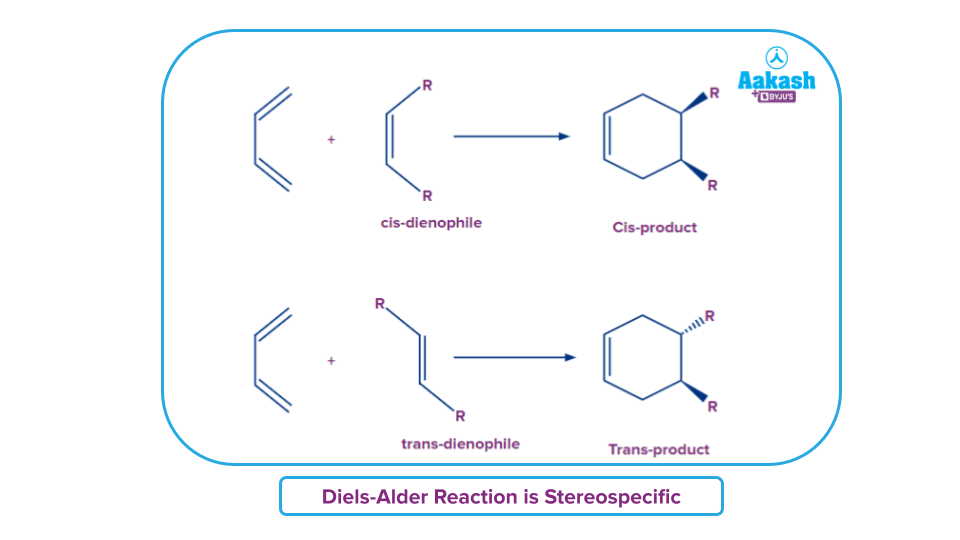
Stereospecific reaction means a reaction whose mechanism dictates that there is no room for choice;and the reaction is specific to the stereochemistry of the starting material. The stereochemistry of the starting material defines the stereochemistry of the final product. The phrase might occasionally be applied to chiral reagents.
The Diels-Alder process is stereospecific with respect to dienophile as well as diene. Synchronised addition for both components (bonds form from the same species at the same time). Due to the planar character of both reactants and the suprafacial nature of the forming process, stereochemistry is retained (i.e. to or from the same face of each plane). This stereospecificity supports Diels-Alder's concerted mechanism.
A Diels-Alder reaction keeps the dienophile's stereochemistry in the final product. When a dienophile is cis, it will produce a cyclohexene ring with cis (syn) substitution on the dienophile's two carbons. In a similar manner, a trans dienophile will produce a cyclohexene ring with trans (anti) substitution on these two carbons.
Both diene substituents land on the same face of the final product if they have the same stereochemistry. If the diene substituents have opposing stereochemistry (for e.g -- one is E and the other Z), they will appear on the product's opposing faces. Depending on whether the dienophile is beneath or away from the diene in the transition state, cyclic dienes can produce stereoisomeric products. Typically, the endo product is the major one (due to kinetic control).
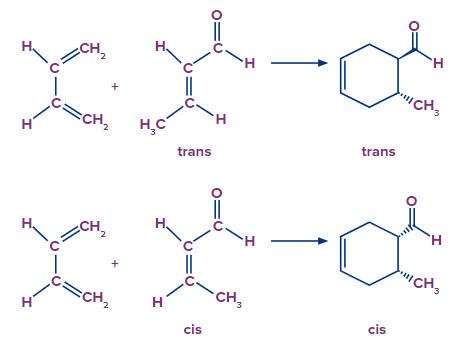
Stereoselectivity: Endo Rule
Stereoselective reaction is a reaction that, although theoretically has the potential to make two or more stereoisomers, but yields only one stereoisomer.
This indicates that stereoselective reactions are those in which among a group of stereoisomers, only one stereoisomer reacts.
The endo rule is followed by Diels-Alder reactions. Let’s understand this rule herein:
- A bridging bicyclic molecule is created in the Diels-Alder process when a cyclic diene is employed.
- There are two possible alignments for the diene and dienophile. The dienophile's electron-withdrawing groups are pointing towards or beneath the diene in one of them. This orientation leads to formation of endo products.
- While the other possible orientation is when the electron-withdrawing substituents (e.g., -NO2, -CN) are pointed away from the diene leading to formation of exo products.
- This is illustrated in the image below.
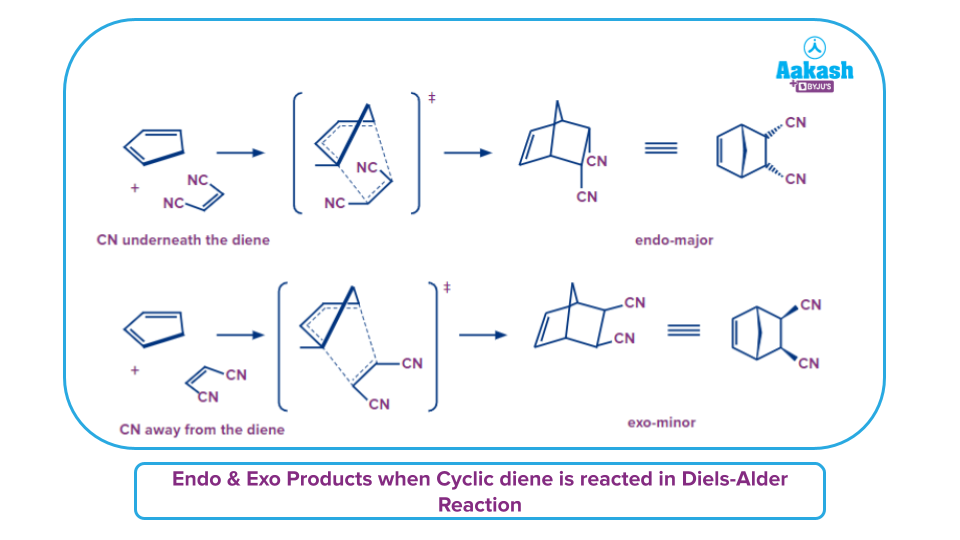
- Usually endo product is more stable as the electron-withdrawing substituents of the dienophile are directed towards the -electrons present in the diene). This results in a beneficial interaction between the non-bonding orbitals of the diene and the dienophile, which lowers the energy of the transition state.
- On the other hand exo is more stable since the substituents on dienophile are directed away from the larger bicyclic ring created.
- Hence Endo is the kinetically controlled product (goes to stable transition state)
- Whereas Exo is a thermodynamically controlled product.
- The instance of kinetic vs. thermodynamic control may be seen in the creation of exo vs. endo.
- Diels-Alder reactions are reversible in nature.
- The exo product is more stable at higher temperatures, but because the activation energy for the endo product is smaller, the endo isomer forms more quickly.
- The less stable endo isomer is the major result when the temperature is lower (transition state reached quickly) because kinetic control is more prominent.
- Higher temperatures and prolonged reaction durations can cause the chemical equilibrium to gain control, resulting in the formation of the thermodynamically more stable exo isomer.
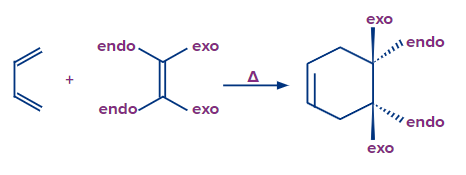
Stereospecific nature of Diels-Alder reaction:
A reaction in which the stereochemistry of the reactants controls the stereochemistry of the product, is called a stereospecific reaction. Typically, one stereoisomer of a specific reactant yields one stereoisomer of a specific product, whereas another stereoisomer of the same reactant yields a different stereoisomer of the same product.
- Reactions of Diels-Alder mechanisms are stereospecific in nature.
- This indicates that during the reaction, the substituents linked to both the diene and the dienophile maintain their stereochemistry.
- For instance, the functional groups on the dienophile should continue to be trans to one another in the products if they were trans to one another in the reactants.
- Hence Trans dienophiles produce rings with trans substitution, whereas cis dienophiles produce rings with cis substitution..
Characteristics of Diels-Alder Reaction
- The reaction is also known as a "cycloaddition" because it results in the production of a cyclic product via a cyclic transition state.
- The Diels-Alder reaction is an electrocyclic process that includes the [4+2] cycloaddition of 2π-electron from the dienophile (an alkene or alkyne) and 4π-electron from the conjugated diene. New bonds, which are energetically more stable than the -bonds are formed during the process.
- It involves an interaction of a 4π- electron system with a 2π-electron system that leads to the Diels-Alder reaction mechanism.
- New σ-bonds, which are energetically more stable than the existing π- bonds are formed during the process.
- As a result of the application of orbital symmetry, this interaction now results in a transition state without any extra energy barrier.
- The diene must possess a s-cis conformation rather than the s-trans conformation.
- The reaction occurs via a concerted mechanism (A chemical reaction is said to be concerted if all bond-forming and bond-breaking takes place in a single step). The Diels-Alder reaction takes place in only one step since it is a concerted reaction. Additionally, all of the atoms involved in the process make bonds at the same time.
- The dienophile's electron-withdrawing groups enhance reaction whereas the diene's electron-donating groups facilitate the reaction.
- The reaction can be inhibited if steric hindrance is present at the bonding sites.
- With regards to the substituent arrangement in both the dienophile and the diene, the reaction is stereospecific.
- The Diels-Alder reaction is the most potent synthetic technique for synthesising unsaturated six-membered rings.
Application of Diels-Alder Reaction
The reaction has been utilised to synthesise several natural chemicals that would be challenging to create otherwise due to its strong stereospecific character. A system's conjugation can be identified using the reaction as a diagnostic tool. Studying the adduct allows one to ascertain the configurations of the reactants because the reaction is stereospecific. The intermediate benzyne has also been trapped using this process. To demonstrate its use, a few responses are provided.
- For the design and synthesis of compounds that were investigated for their antiviral activity against a variety of viruses, Diels-Alder and their hetero variant were used.
- A review of 1,3-dipolar cycloaddition reactions of a few 1,3-dipoles, including azides, nitrones, and nitrile oxides, is also provided in light of their use in the creation of crucial intermediates for the synthesis of antiviral drugs as well as certain anti-cancer drugs.
- It has been documented that functionalized acyclic dienes may be used in Diels-Alder reactions with arynes to create valuable cis-substituted dihydronaphthalene building blocks.
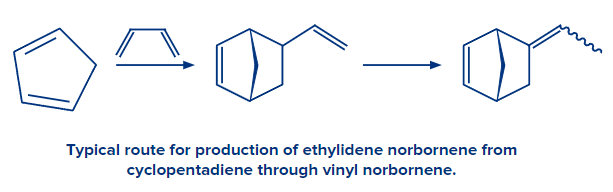
- The industrial manufacture of cyclopentadiene employs the retro Diels-Alder reaction.
- The precursor of several norbornenes, which are typical monomers, is cyclopentadiene.
- The manufacture of vitamin B6 also uses the Diels-Alder process.
- Diels-Alder reactions are useful for the synthesis of: Natural and Synthetic poly heterocycles and polycarbocycles.
- It is used in the manufacture of several alkaloids, cyclopenta quinoline rings, and substituted tetrahydroquinoline, substituted anthraquinone derivatives, quinolones.
- Analysis has been done on the chemical thermodynamics of the Diels-Alder addition reactions of a number of acenes (such as anthracene and pentacene) to C60 fullerene.
- Without utilising a catalyst, cross-linked hydrogels have been created via the Diels-Alder process.
- Graphite has been mechanically exfoliated into graphene adducts using the Diels-Alder process with tetracyanoethylene.
- It is applied in forming cantharidine. The starting material for this challenging synthesis of natural cantharidine is the Diels-Alder adduct formed from luran and acetylene dicarboxylate.
Practice Problems
Q.1. The Diels Alder Reaction follows a concerted mechanism. This means:
- All bond making and bond breaking occurs simultaneously
- The reaction is endothermic
- The product contains rings
- The reaction follows Markonikov’s rule
Answer: The process is a one-step cycloaddition reaction because the Diels-Alder reaction mechanism is concerted. Here, a cyclic adduct is created when two unsaturated molecules come together. Bond multiplicity has decreased overall. Bond creation and bond destruction all take place at once. So Option A is correct.
Q.2. Diels-Alder Reaction called a cycloaddition reaction of the type?
- [4+2]
- [2+2]
- [1+3]
- [1+2]
Answer: In particular, only six-membered rings are produced via the Diels-Alder process. The reaction needs a diene (which has a 4π-electron component) and a dienophile (which has a 2π-electron component). Because of this, the Diels-Alder is frequently referred to as a [4+2] cycloaddition. So option A is correct.
Q.3. The diene should assume — conformation during Diels Alder reaction?
- Transoid
- Cisoid
- Doesn’t matter
- None of the above
Answer: The two C-C π-bonds must adopt a conformation where they are in the same plane rather than just being near to one another like diene's pi-bonds, which is insufficient (i.e. flat). The diene must be in the "s-cis" conformation in order to prevent the two responding ends from being too far apart. Two double bonds that are on the same side of a sigma bond are said to be in the "s-cis" conformation. So Option B is correct.
Q.4. What is endo-exo selectivity?
Answer: The two reactants namely diene and dienophile, enter the system one on top of the other since p orbitals are involved. There are two ways the dienophile might approach the diene. The direction of the electron withdrawing group can either be endo—straight above or below the diene—or ecto—away from the diene (exo). In terms of thermodynamic stability, the exo product is typically superior. Because the endo transition state has less energy from a kinetic perspective, the endo product forms more quickly.
Frequently Asked Questions–FAQs
Q.1. Is Diels Alder reactions endothermic or exothermic?
Answer: The Diels Alder reaction is an exothermic reaction. The reaction releases heat as a result of the creation of new C-C bonds. The transformation of two weak -bonds into two stronger -bonds cause this reaction to be exothermic.
Q. 2. Why does the Diels-Alder reaction occur?
Answer: A more stable chemical is produced by the Diels-Alder reaction, which is a cycloaddition of a 4 pi + 2 pi (diene + dienophile) system. This is because the newly generated sigma bonds are more stable than the pi bonds that were previously present.
Q. 2. Why is Diels-Alder a syn addition reaction?
Answer: An extra reaction in which all new bonds are created on the same face of the reactant molecule. Because the two new carbon-carbon sigma bonds are produced on the same face of the diene or dienophile, this Diels-Alder reaction is a simultaneous cycloaddition process.
Q. 4. What distinguishes a diene from a dienophile?
Ans: A diene is an unsaturated hydrocarbon with two double bonds and is conjugated, whereas a dienophile (non-conjugated) is an organic substance that easily interacts with a diene. This is the main distinction between the two.


