-
Call Now
1800-102-2727
Structure and Preparation of Diazonium Salt - Diazonium Salt, Structure, Stability, Preparation and Physical Properties of Diazonium Salt, Practice Problems, FAQs
An advertisement claimed a versatile product that can chop, cut, slice, shred, grind. knead, beat, and squeeze easily and quickly. Guess what it is? Or ask your mother. Yes it is the one food process that has multiple task capability and yield different products of our choice.
Like a food processor there are certain organic compounds that are capable of forming many other organic compounds of interest. Grignard reagents and Diazonium salts are two examples of such versatile reagents in organic preparations.
You shall learn about the diazonium salt and its versatility below.
Table of content
- Diazonium Salt
- Structure and Stability of Diazonium Salts:
- Preparation of Diazonium Salt
- Physical Properties of Diazonium Salt
- Practice Problems
- Frequently Asked Questions
Diazonium salt
Diazonium salts are ionic salts having a general formula, where
. Diazo indicates the presence of two nitrogen atoms linked to each other.
The suffix diazonium is appended to the name of the aromatic hydrocarbon from which they are derived, followed by the name of the anion. As an example,
(Benzene diazonium chloride)
The is called diazo group.

Diazonium salts serve as useful synthetic intermediates for the synthesis of a variety of aromatic compounds and azo dyes.
Structure and stability of diazonium salts:
The diazonium salts have a positively charged diazo group (-N+ = N) which is quite unstable.
An aliphatic alkyl group will not be able to stabilize much the positive charge. Primary aliphatic amines form highly unstable alkane diazonium salts. Even at low temperatures, they decompose quickly (< 273 - 278 K) forming carbocation (R+) and nitrogen gas.
R - N+ = NX- -> R+ + N2 + X-
On the other hand, an aryl attachment to nitrogen can help the positive charge to get dispersed inside the benzene ring by resonance and stabilizing the diazonium salt. The dispersal of the positive charge over the benzene ring gives arenediazonium salts their stability, as shown below:
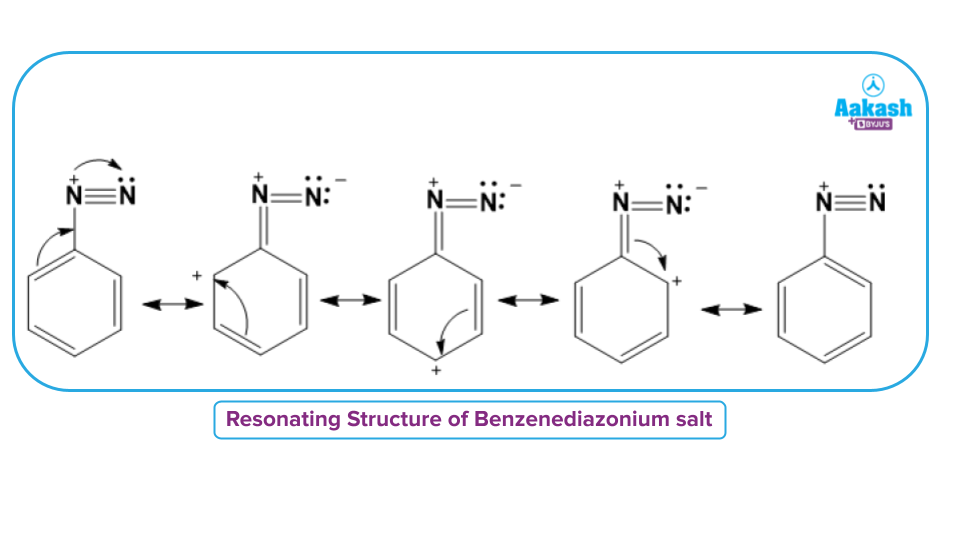
Arene diazonium salts, are stable for a short time in solution at low temperatures (273-278 K). Thus, aromatic diazonium (arenediazonium) salts are much more stable than aliphatic diazonium (alkane diazonium) salts.
The instability of alkane diazonium salts is due to their larger tendency to form carbocations by removing an extremely stable nitrogen molecule.
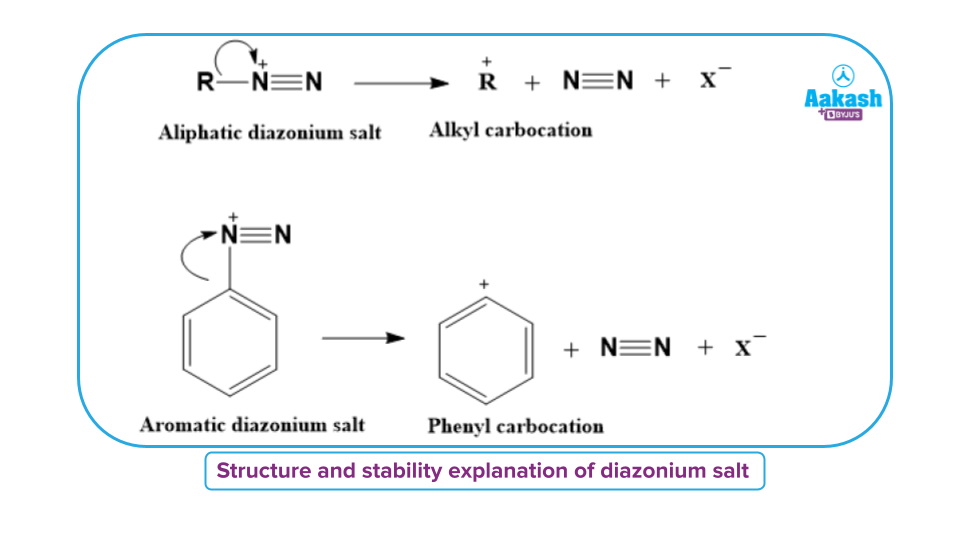
Since the phenyl (or aryl) carbocation is much less stable than alkyl carbocations, therefore, aromatic diazonium salts have a much lower tendency to eliminate nitrogen than aliphatic diazonium salts. Aromatic diazonium salts, on the other hand, are much more stable than aliphatic diazonium salts.
Preparation of diazonium salt
Aromatic diazonium salts are generally prepared by adding a cold aqueous solution of sodium nitrite to the solution or suspension of a primary aromatic amine in an acid medium maintained at around 273 - 278 K.

Diazotisation is the process of converting a primary aromatic amine into its diazonium salt.
General procedure for diazotisation
The primary aromatic amine is dissolved or suspended in a dilute acid such as HCl, H₂SO4, etc. (Three moles of the acid are generally used for every mole of the amine to be diazotised; one mole to form the salt of the amine, one mole to liberate nitrous acid from sodium nitrite and one mole to keep the reaction mixture acidic enough to suppress the undesirable side reactions such as coupling of the diazonium salt thus formed with the free amine).
The amine solution is cooled and a cold aqueous solution of sodium nitrite is added slowly to it at such a rate that the temperature of the reaction mixture does not rise above 278 K. The addition of sodium nitrite solution is stopped as soon as a few drops of the reaction mixture produces a blue colour with starch-potassium iodide paper showing the presence of unreacted nitrous acid in the reaction mixture.
Too much excess of nitrous acid is generally avoided since it subsequently interferes with the reactions of diazonium salts. The excess of nitrous acid may be destroyed by the addition of urea.
Since diazonium salts slowly decompose even at low temperatures (273-278 K), they are used immediately after preparation.
Mechanism.
The diazotisation of amines is believed to occur by the following mechanism.
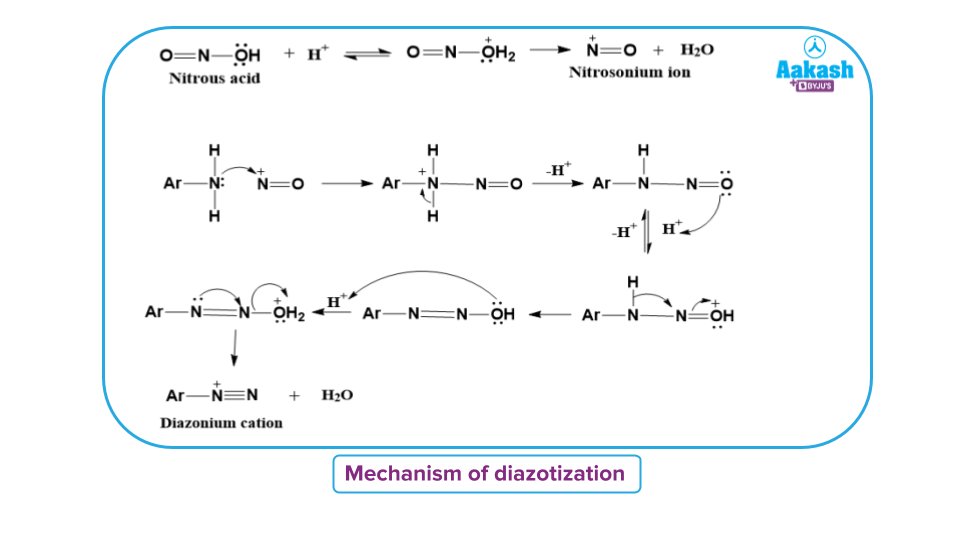
This mechanism is supported by the observation that aromatic amines contain electron-withdrawing groups such as -NO₂ are less easy to diazotise. This is due to the reason that nucleophilicity of the amino group is reduced due to the strong electron-withdrawing effect of the - group as shown below:
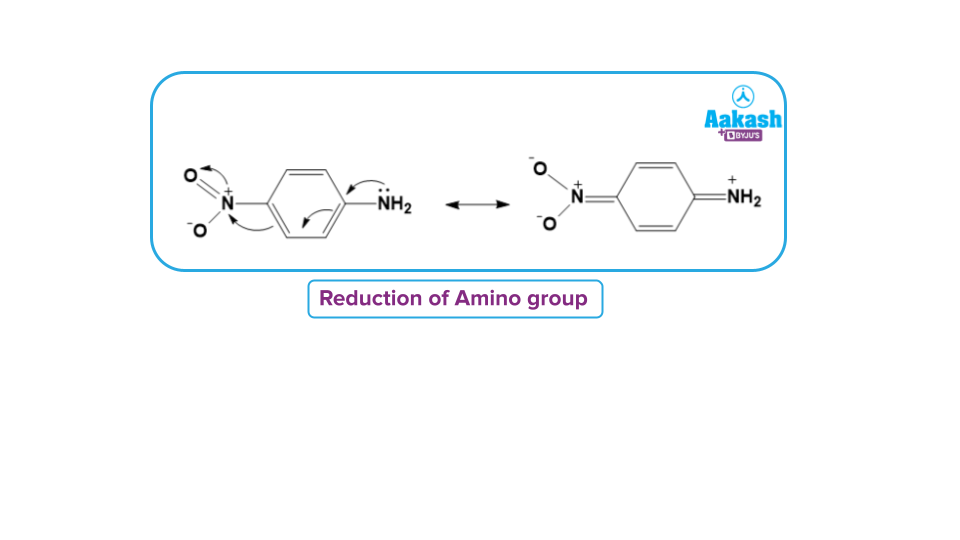
Such amines can, however, be diazotised in acetic acid.
Conversely, aromatic 1° amines containing electron-donating groups at o- and p-positions (e.g. o-toluidine p-anisidine, etc.) undergo diazotisation much more readily than aniline.
Physical properties of Diazonium Salt
Arenediazonium salts are generally colourless ionic crystalline solids. It is highly soluble in water at low temperature, but may react at temperatures above 278 K.. Many diazonium salts especially nitrates are dangerously explosive in the dry state. Therefore, they are never isolated but usually prepared in situ and used immediately after their preparation.
However, certain diazonium salts such as fluoroborates are relatively insoluble in water and are stable enough to be dried and stored. Some diazonium salts also form complexes with metallic salts such as chloride e.g. (ArN2)2 ZnCl24-. These complexes are generally insoluble.
Practice Problems
Q1. Which of the following diazonium salts RN2+X- will be the most stable ?
Answer: (B)
Solution: The conjugation of the N = Ntriple bond with the benzene ring makes the benzene diazonium halide the most stable of the provided diazonium salts.
Q2. Which of the following is not a diazonium salts property?
- Diazonium salts are colourless crystalline solids.
- They are soluble in water because they are ionic in nature.
- When dried, the majority of these salts explode.
- Diazonium salts are poor conductors fo electricity under aqueous conditions.
Answer: (D)
Solution: Because the ions in salt solutions like sodium chloride (NaCl) have the freedom to move around in solution, they can conduct an electric current. When sodium chloride dissolves in pure water, sodium (Na+) and chloride (Cl-) ions are created.
Salts of diazonium () Due to the ions present, aqueous solutions are neutral to litmus and conduct electricity. The ions and electrons are much more mobile in an aqueous solution, allowing electricity to flow through the solution.
Q3. Which of the following statements about the property of benzenediazonium fluoroborate is correct?
- At room temperature, it dissolves in water.
- It is insoluble in water and remains stable at room temperature.
- It is a coloured solid.
- None of the above
Answer: (B)
Solution: The compound benzenediazonium fluoroborate is not water soluble. At room temperature, it is comparatively stable. We can infer from the aforementioned characteristics that option B is the correct response to this question.
Q4.What degree of temperature is ideal for the diazotization reaction to occur?
- 273K
- 283K
- 298K
- 303K
Answer: (A)
Solution: At extremely low temperatures, aniline and nitrous acid react to form benzene diazonium salts (273-278K). Phenol will form if the temperature rises above 278 K.
Hence, option A is correct answer.
Frequently Asked Questions (FAQs)
Q1. Why is the diazotisation reaction kept at such a low temperature?
Answer: Because diazonium salts form other materials at high temperatures and provide phenol by reacting at high temperatures with water, we must keep the temperature low during diazotization. Otherwise, we will make a major error in the experiments. At high temperatures, aryldiazonium salts decompose into hydrogen chloride, nitrogen gas, and chlorobenzene. The salts, on the other hand, are never completely dry and are kept at extremely low temperatures.
Q2. Why do we add excess mineral acid in diazotisation reaction?
Answer: This is done to keep the mixture strongly acidic, which is required to prevent benzene diazonium chloride from coupling with too much aryl amine.
Q3. Can we dye our fabrics using diazonium salt?
Answer: Yes, as they are used in the dye and pigment industries, as well as in the production of dyed fabrics. But you this process can only be carried out in a suitable laboratory condition
Q.4 What makes diazonium salts so reactive?
Answer: Diazonium salts are highly reactive as they can violently disintegrate when subjected to heat or mechanical force (shock-sensitive) due to their high energy. The reaction enthalpy of the diazo functional group ranges from -160 kJ/mol to -180 kJ/mol.


