-
Call Now
1800-102-2727
Detection of Halogens– Lassaigne’s Test for Detection of Halogens, Carius Method for Quantitative Analysis of Halogens, Practice Problems & FAQs
Investigation has always been an integral part of chemistry and chemical assay. To find out the presence or absence of a certain thing has always been an enthralling step towards chemical analysis and has helped immensely in the field of scientific and industrial growth at large.
In synthetic organic chemistry, halogens are integrated into molecules called organohalides. It is frequently used in high-intensity discharge lamps called metal halide lamps. They are used as a complement to natural sunshine in greenhouses or wet climates.

In the pharmaceutical sector, organo halides are crucial. Drugs come in a wide variety of organohalide forms. It has been suggested that trifluoromethyl and difluoromethyl groups can increase pharmacological action. Similar to how they are used in the chemical industry, these substances are also used as plasticizers, insecticides, elastomers, adhesives, sealants, coatings that are electrically insulating, solvents, pesticides, refrigerants like freon, fire-resistant oils, and pesticides. Halo alkenes were used as some of the chemical building blocks for plastics like teflon and polyvinyl chloride (PVC, also known as polymerized chloroethene).
Derivatives of organic compounds in which one or more hydrogen atoms have been replaced by an equal number of halogen atoms are known as organic halogen compounds (F, Cl, Br, or I). Halogen atoms can be found in almost every category of organic compounds, including alcohols, ketones, and carboxylic acids. Hence it is immensely important to study ways and methodologies that are employed in laboratories to detect the presence of halogens in any given organic compound. Let’s find them out!
Table of Contents
- What is the Lassaigne Test?
- Preparation of Sodium Fusion Extract
- Lassaigne Test for Detection of Halogens
- Carbon Disulphide Test for Bromine and Iodine
- Beilstein Test
- Carius Method for Quantitative Assay of Halogens
- Practice Problems
- Frequently Asked Questions-FAQs
What is the Lassaigne Test?
Lassaigne’s Test is a very useful method of detection of halogens in organic compounds. This test is used to determine whether an organic compound contains halogens, nitrogen, or sulphur.
The organic substances are covalently joined to these elements. These must be changed into their ionic forms in order to be detected. By fusing the organic chemical with sodium metal, this is accomplished. Simple chemical assays can be used to identify the ionic chemicals created during the fusion process, which are extracted in aqueous solution. The extract is also known as Lassaigne's extract or sodium fusion extract. The elements present in a compound are converted from covalent to ionic form by fusing the organic compound with Sodium (Na) metal.
The test involves two steps: Preparation of the Sodium-fusion extract and detection of the elements using it.
Preparation of Sodium Fusion Extract
In a fusion tube, a tiny amount of an organic molecule is fused with a tiny amount of sodium metal. Cyanides, sulphides and halides of sodium formed on sodium fusion are extracted from the fused mass by boiling with water.
The fusion tube is then submerged in distilled water, its contents are cooked for a short period of time, then they are cooled, filtered and chilled. The filtrate is known as Lassaigne's extract or sodium fusion extract. Typically, it is alkaline. A few drops of NaOH solution can be added to make it alkaline if it isn't already. The resulting sodium fusion extract is used to identify elements like N, S, Cl, Br, and I.
An experiment for the preparation of sodium fusion extract (Lassaigne’s extract) is given below in two parts.
Step 1:
In an ignition tube, a very little quantity of dry sodium metal is heated until it resembles a shiny ball. The to-be-tested organic chemical is then added, and the mixture is heated once again until the ignition tube becomes extremely hot. Distilled water is taken and placed in a china dish. The contents of the ignition tube and the tube are then added.
Step 2:
To stop the dish from bursting, wire gauze is immediately placed on top of it. To acquire Lassaigne's extract, the contents of the china dish are heated once again until they are reduced to one-third of their original volume.

To make organic molecules that have covalent connections with the elements nitrogen, sulphur, and halogens more easily detectable, sodium is used in a reaction.
During the fusion reaction, the components in the organic complex interact with sodium as follows:
Where, X= Cl, Br, I.
Lassaigne Test for Detection of Halogens
When halogens in an organic substance fuse with sodium metal, they produce sodium halide. After acidifying with dilute HNO3, sodium halide extracted with water can be quickly detected by adding silver nitrate solution.
- A white curdy precipitate that is soluble in ammonium hydroxide solution forms when chlorine is present in the sample.
- Bromine is present because it produces a pale yellow precipitate that is only partially soluble in NH4OH.
- While the presence of iodine in the organic compound is confirmed by the production of a bright yellow precipitate that is insoluble in NH4OH.
To summarise this:
|
Colour of Precipitate |
Solubility in NH4OH |
Halogen |
|
White |
Completely Soluble |
Chlorine |
|
Pale Yellow |
Sparingly Soluble |
Bromine |
|
Bright yellow |
Insoluble |
Iodine |
Carbon Disulphide Test for Bromine and Iodine
To break down sodium cyanide and sodium sulphide, a tiny amount of the Lassaigne's extract is heated with dilute H2SO4. After cooling, a few millilitres of freshly made chlorine water, carbon disulfide, or carbon tetrachloride are added to the solution. When the mixture is shook ferociously and left to stand.
- The presence of bromine is indicated by the CS2 or CCl4 layer turning orange.
(Dissolves in CS2 or CCl4 to give orange colour)
- Violet coloured layer in CS2 or CCl4, indicates the presence of iodine.
(Dissolves in CS2 or CCl4, to give violet colour)
This test is known as organic layer test. Here, iodine and bromine are expelled from their equivalent halides by chlorine. Thus released halogen dissolves in CS2 or CCl4 to provide the desired colour.
Beilstein Test
An easy qualitative chemical test for organic halides is the Beilstein test. In this test, a clean, sturdy copper wire is heated in the Bunsen burner's non-luminous flame until it stops giving the flame any green or bluish green colour. The heated end is again placed into the Bunsen flame after being immersed in the organic substance. When volatile cupric halides develop and emit a green or bluish flame, this indicates the existence of halogens in the organic molecules. The test doesn't detect fluorides.
Limitations of Beilstein test:
- This is also caused by the creation of volatile cupric cyanide, which occurs in organic molecules like urea, thiourea, etc. that do not include halogens.
- Which halogen—iodine, bromine, or chlorine—is truly present in the organic compound cannot be determined.
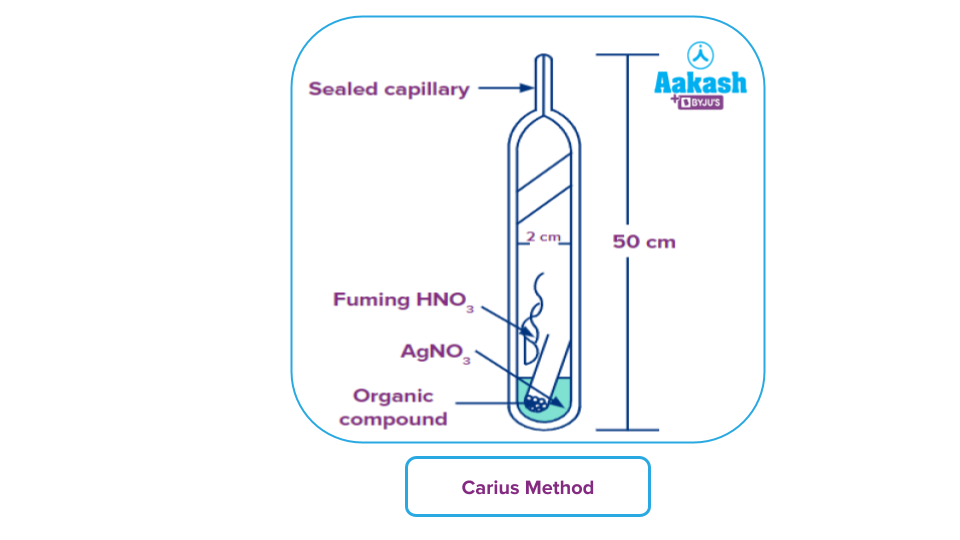
Carius Method for Quantitative Assay of Halogens
An analytical chemistry technique for halogen quantification in chemical compounds is the Carius halogen method.
In a furnace, a known mass of an organic compound is heated with fuming nitric acid (HNO3) while silver nitrate (AgNO3) is present inside a carius tube made of strong glass. The compound's carbon and hydrogen are converted to carbon dioxide and water through oxidation. The silver halide that corresponds to the halogen present is formed (AgX). It is cleaned, dried, filtered, and weighed.
Percentage of X =
Percentage of Cl =
Percentage of Br =
Percentage of I =
Practice Problems
Q.1. In order to check for halogens, the Lassaigne's extract is boiled with concentrated HNO3. As a result, it:
- Decomposes NaCN and Na2S if formed.
- Assists in the precipitation of AgCl.
- Increases the Ksp of AgCl.
- Increase the concentration of ions.
Answer: (A)
Solution: Before testing for halogens, sodium extract (Lassaigne's extract) is boiled with diluted HNO3 to break down any NaCN or Na2S present, as doing so would cause them to generate white ppt with AgNO3 and interfere with the halogen test.
So, the correct answer is option (A).
Q.2. Why is it not possible to detect fluoride ion via Lassaigne Test?
Answer: Since AgF is soluble in water, it is impossible to detect the presence of fluorine using this test. In fact it is difficult to devise a safe method of fluorine detection at the laboratory. Highly efficient and skilled methods are employed.
Q.3. Determination of chlorine is possible in which of the following compounds, without preparing sodium fusion extract?
- CHCl3
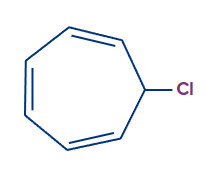
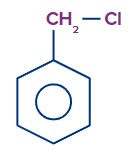
Answer: (C)
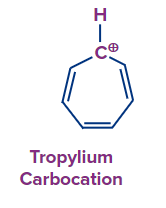
Solution: The compound that naturally ionises to give chloride ion and forms stable conjugate cation, has the capacity to give positive chloride test using (white AgCl ppt) with AgNO3. Tropylium chloride forms tropylium cations which are highly stable due to its aromaticity. It is cyclic, conjugated and has electrons. Hence, the correct answer is option C.
Q.4. Using Carius method for estimation of halogens, 0.17g of an organic compound gave 0.13g of AgCl. Percentage chlorine in the compound is:
- 35 %
- 87 %
- 54.4 %
- 18.9 %
Answer: (D)
Solution: Mass of organic compound = 0.17 g
Mass of AgCl = 0.13 g
Molar mass of AgCl = 143.5 g mol-1
Putting all the values in the formula discussed:
Percentage of Cl = 3
So, option D is the correct answer.
Frequently Asked Questions-FAQs
Q.1. Why is the sodium fusion extract alkaline?
Answer: In addition to the release of hydrogen gas, the excess sodium interacts with the water to produce hydroxide ions. Hence, it is alkaline.
Q.2. How can we test for fluoride in water samples?
Answer: Fluoride testing is difficult to perform in households or in laboratories. But Bhabha Atomic Research Centre (Indian) has devised a method for doing this.
At the Bhabha Atomic Research Centre in India, fluoride measurement is based on the bleaching of zirconium xylenol orange complex. Moreover, the kit includes a colour chart with a range of 0–3 ppm. The user must combine a 4 ml water sample with 1 ml of zirconium xylenol orange reagent to conduct the test. Depending on the amount of fluoride in the sample, the colour shifts from pink to yellow. The amount of fluoride present in the water can be determined by comparing the colour that results with a colour chart. Due to human inaccuracy in visual judgement, it is challenging to estimate the concentration accurately. Furthermore, it can be challenging to measure liquid quantities correctly in real-world settings, which increases error.
Q.3. How is the trace amount of fluoride measured?
Answer: The main techniques used to measure trace amounts of fluoride in biological fluids are potentiometric (Ion Selective Electrode [ISE]) and gas chromatographic (GC) techniques.
Q.4. Why is the Beilstein test usually not used for detecting halogens?
Answer: It is not commonly used since, if polychloroprene is employed as the test material, it is possible to produce the extremely dangerous chloro-dioxins. Dioxins, also known as polychlorinated dibenzodioxins (PCDDs), are a class of harmful, long-lasting organic pollutants that are predominantly anthropogenic and contribute to persistent organic pollution in the environment.


