-
Call Now
1800-102-2727
General Features of d-block Elements - Introduction, The Position of d-block Elements in the Periodic Table, General Features, Practice problems, FAQs
d block elements- Introduction, Position in the periodic table, General Properties of d block, Colour imparting tendency by d block elements, Practice Problems, Frequently asked questions-FAQs
Heena and Rekha are two sisters, they were going shopping for their brother’s marriage. We all know how excited people are if there is a wedding in their family. Along with Heena and Rekha, Amit, their younger brother who is studying in class 12 is also giving them company while shopping.
After buying clothes and sweets it was time for Heena and Rekha to buy jewellery. They went to a jewellery shop to buy different jewellery, all of them very shiny. Amit was sitting behind and was thinking about the elements of which this jewellery was made.
After returning home, everyone was busy preparing for marriage but Amit was thinking about only jewellery and what elements are present inside them. Let us help him out in finding his solution.
Wait! We are not here to imagine what Amit was thinking, but we are here to give that answer. Generally, jewellery are made up of precious metals like silver, gold, and platinum. These are very expensive in nature. They have many other properties also. Why I am telling this to you? They all belong to the d block elements which we are going to discuss now.
From the third group of the modern periodic table to the twelfth group, there are elements which are known as d block elements. There are many elements which have multiple applications and they play significant roles in chemical reactions.
Let's discuss their properties, position and applications in detail.

TABLE OF CONTENT
- What are d-block elements?
- Position of d block elements in the periodic table
- Electronic configuration of d block elements
- General Properties of d block elements
- Colour imparting tendency by d block elements
- Practice Problems
- Frequently asked questions-FAQs
What are d-block elements?
Filling the 3d, 4d, and 5d electron shells results in the formation of three series of elements. The d-block components are made up of all of those series. Because of their location in the periodic table between the s-block and p-block elements, they are frequently referred to as "transition elements." They exhibit transitional qualities between the extremely reactive metallic elements of the s-block, which frequently form ionic compounds, and the predominantly covalent elements of the p-block. The outer shell of the atom is expanded with electrons in the s- and p-blocks. The penultimate shell in the d-block expands from 8 to 18 electrons as a result of the addition of electrons.
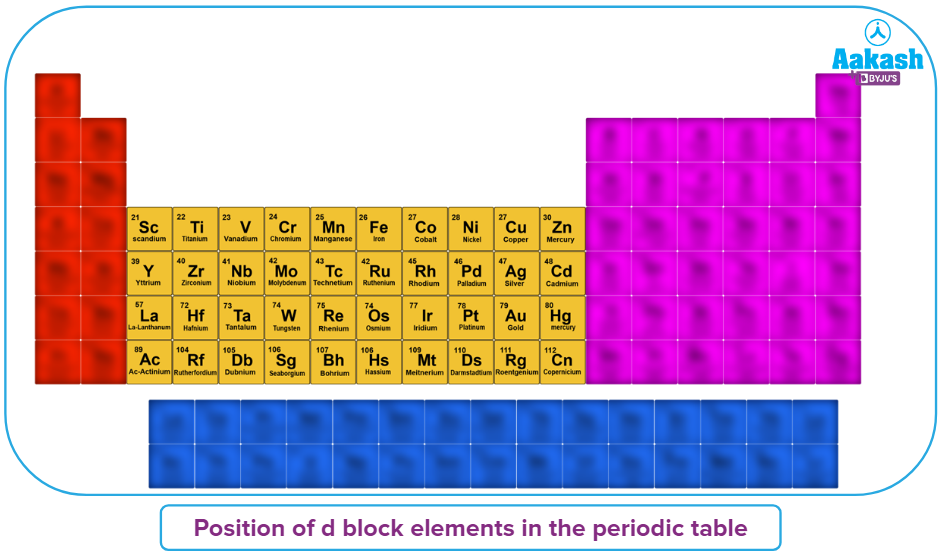
Position of d-block elements in the Periodic table
The d-block is located in the middle of the periodic table, between the s and p blocks. The d-block components are only given the moniker "transition" because of their location between s- and p-block elements. The transition metals' three rows, 3d, 4d, and 5d, are created when electrons are taken up by the d-orbitals of the penultimate energy level in their atoms. Below is the periodic table which depicting the position of d block elements.
Electronic configuration of d block elements
Electrically, these parts are typically configured as refers to the inner d orbitals, which have an electron count range of one to ten, and the outermost ns orbitals, which have an electron count range of one to two. This generalisation includes numerous exceptions because the energy gap between the (n-1)d and ns orbitals is so narrow. Furthermore, orbital sets with partial and complete fillings are more stable. The electronic configurations of Zn ,Cd, and Hg are represented by the general formula(n-1)d10ns2 . These elements' orbitals are completely filled in both their ground state and regular oxidation states. They have not taken into account transitional features as a result.
Electronic configuration of d block elements are mentioned below:
3d series
|
Element |
Symbol |
Electronic configuration |
|
21 |
Sc |
|
|
22 |
Ti |
|
|
23 |
V |
|
|
24 |
Cr |
|
|
25 |
Mn |
|
|
26 |
Fe |
|
|
27 |
Co |
|
|
28 |
Ni |
|
|
29 |
Cu |
|
|
30 |
Zn |
4d series
|
Atomic number |
Element |
Electronic configuration |
|
39 |
Y |
|
|
40 |
Zr |
|
|
41 |
Nb |
|
|
42 |
Mo |
|
|
43 |
Tc |
|
|
44 |
Ru |
|
|
45 |
Rh |
|
|
46 |
Pd |
|
|
47 |
Ag |
|
|
48 |
Cd |
5d series
|
Atomic number |
Symbol |
Electronic configuration |
|
57 |
La |
|
|
72 |
Hf |
|
|
73 |
Ta |
|
|
74 |
W |
|
|
75 |
Re |
|
|
76 |
Os |
|
|
77 |
Ir |
|
|
78 |
Pt |
|
|
79 |
Au |
|
|
80 |
Hg |
All of the series do experience some anomalies, which the following ideas can help to explain these anomalies.
- The difference in energy between the (n-1)d and (ns) orbitals.
- Pairing energy of the electrons present in the s orbital as compare to d orbitals
- Stability of partially filled orbitals relative to those of fully filled orbitals.
Copper has an electron configuration of as opposed to and for chromium it is . The stability of half-full orbitals in comparison to partially filled orbitals helps to explain the inconsistencies in the first transition series.
The existence of electrons in d orbitals rather than shared s orbitals seems to be preferred in the second series of transition metals starting with niobium. The electron has a choice of sharing in the s orbital or being stimulated to the d orbital among the available s and d orbitals. The energy difference between the s and d-orbitals and the repulsive energy it has overcome on sharing are obviously factors in the decision and results to the configuration of Nb.
Because the energy of the s and d orbitals in the second series is nearly equal, electrons choose to occupy the d orbital. Therefore, the s-orbital of niobium possesses primarily one electron. On the other hand, third series transition metals have more paired s configuration despite having less half-filled orbitals (Tungsten- ). This series follows the contraction of the lanthanide caused by the filling up of 4f orbitals.
General Properties of d block elements
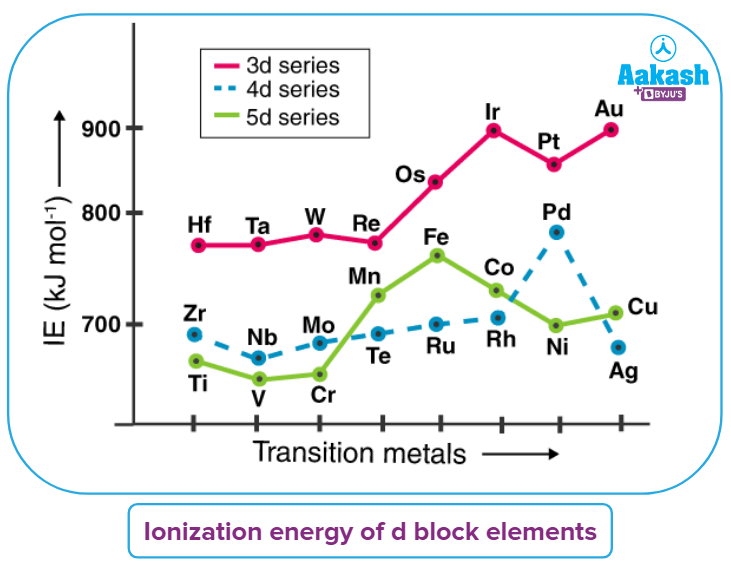
Ionisation energy of d block elements:
Ionization energy, which is closely correlated with the force of attraction on the electron, is the energy required to remove the valence electron from the atom/ion. Ionization energy will therefore increase as nuclear charge increases and electron radii decrease (IE). Ionization Energy will also be higher for orbitals that are partially and completely filled.
Ionization The energy of the d-block elements, between which they are positioned, is greater than that of the s-block and lower than that of the p-block elements. With the exception of chromium and copper, the first Ionization Energy in the first series entails removal from a filled s-orbital. The ionisation energy of the d block elements among them rises as the atomic number grows up to Fe.
Due to a rise in nuclear charge brought on by the filling of the inner d orbitals, the ionisation enthalpy increases along each series of transition elements from left to right. There are countless slight variations, though.
The uneven trend in the first ionisation enthalpy of 3d metals, though of modest chemical significance, can be explained by observing how the removal of one electron alters the relative energies of 4s and 3d orbitals. As a result, the unipositive ions lack 4s electrons and have dnconfigurations. There is an increase in exchange energy as well as reorganisation energy associated with ionisation when the number of electrons increases and s electrons moves from s orbitals into d orbitals. The number for Cr is lower because there is no change in the d configuration, while the value for Zn is larger because it represents ionisation from the 4s level.
Oxidation state:
One of the distinguishing characteristics of a transition element is the wide range of oxidation states that it can exhibit in its compounds.
Transition Metal Oxidation States in the First Row (the most common ones are in bold types)
|
Sc |
Ti |
V |
Cr |
Mn |
Fe |
Co |
Ni |
Cu |
Zn |
|
+3 |
+2 +3 +4 |
+2 +3 +4 +5 |
+2 +3 +4 +5 +6 |
+2 +3 +4 +5 +6 +7 |
+2 +3 +4 +6 |
+2 +3 +4 |
+2 +3 +4 |
+1 +2 |
+2 |
Transition metals oxidation states in the second row (the most common ones in bold types)
|
Y |
Zr |
Nb |
Mo |
Tc |
Ru |
Rh |
Pd |
Ag |
Cd |
|
+3 |
+2 +3 +4 |
+3 +5 |
+1 +3 +4 +5 +6 |
+4 +6 +7 |
+2 +3 +4 +6 |
+2 +3 +4 |
+2 +4 |
+1 +2 |
+2 |
Transition metals oxidation states in the third row (the most common ones in bold types)
|
La |
Hf |
Ta |
W |
Re |
Os |
Ir |
Pt |
Au |
Hg |
|
+3 |
+4 |
+5 |
+1 +4 +5 +6 |
+3 +4 +6 +7 |
+4 +6 |
+3 +4 |
+2 +4 |
+1 +3 +5 |
+1 +2 |
Metallic character
d block elements crystallise in bcc/ccp/hcp structures and exhibit typical metallic properties such as strong tensile strength, malleability, ductility, electrical and thermal conductivity, and metallic lustre.
Except for Copper, they are extremely hard, have a high enthalpy of atomization, and little volatility. The quantity of unpaired electrons correlates positively with hardness. This makes the metals Cr, Mo, and W among the d block elements exceedingly hard. This is likewise the case with the group-12 elements (Zn, Cd, and Hg).
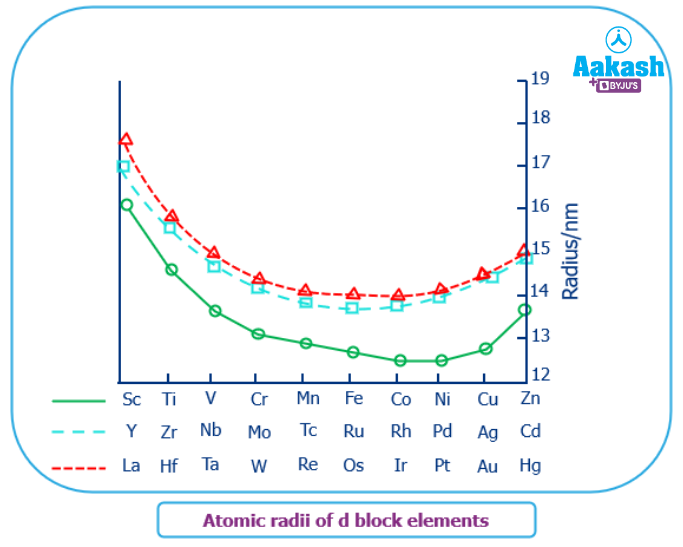
Atomic Radius:
Across a period: Due to increased nuclear charge, the atomic radii of representative (A group) elements decrease dramatically as we read across a period of elements. In the transition series, however, the decrease in atomic radii is not constant because electrons are added to an inner d subshell, which effectively shields the outer electrons, and thus the outer s electrons are not pulled closer, resulting in a very small decrease or relatively constant size as nuclear charge shielding increases.
Across a group: As you progress along with the group, you'll see an increase in the atomic and ionic radii of the elements. The presence of a greater number of subshells can explain the rise in radius.
Magnetic Properties of d block elements
Depending on how they interact with the magnetic field, materials can be categorised as:
- Diamagnetic: Electrons that are coupled, or that lack unpaired electrons, are what define
diamagnetic substancces.
- Paramagnetic: The majority of atoms with partially filled atomic orbitals are paramagnetic because the presence of unpaired electrons in the material causes paramagnetic behaviour.
- Ferromagnetic: Atomic electrons in ferromagnetic elements are organised into domains, each of which has a single charge. These domains align in the presence of a magnetic field, resulting in parallel charges throughout the entire compound.
Orbital magnetic moment and spin magnetic moment are both influenced by unpaired electrons. However, for the third series, the orbital angular moment is insignificant, and the approximation of the magnetic moment due to spin alone is provided by the following formula:
where n represents the number of unpaired electrons present.
Bohr Magneton is its unit (BM). For higher d-series, in addition to the spin moment, the actual magnetic moment also contains the orbital moment. The largest number of magnetic moments and unpaired electrons (six) are found in chromium and molybdenum.
Colour imparting tendency by d block elements:
A substance's colour is caused by the electromagnetic spectrum's visible region (4000 to 7000 A) wavelengths that it absorbs while transmitting or reflecting the remainder. A particular colour is represented by a different wavelength of visible light. All colour combination is present in white light.
The majority of transition metal compounds are coloured when they are in their solid or solution phases. The excitation of electrons from lower energy d-orbitals to higher energy d-orbitals is what gives transition metal ions their colour. The visual range has the energy needed for d—d electron excitations. This explains why transition metal ions have the ability to absorb specific visible radiations and display complementary colour.

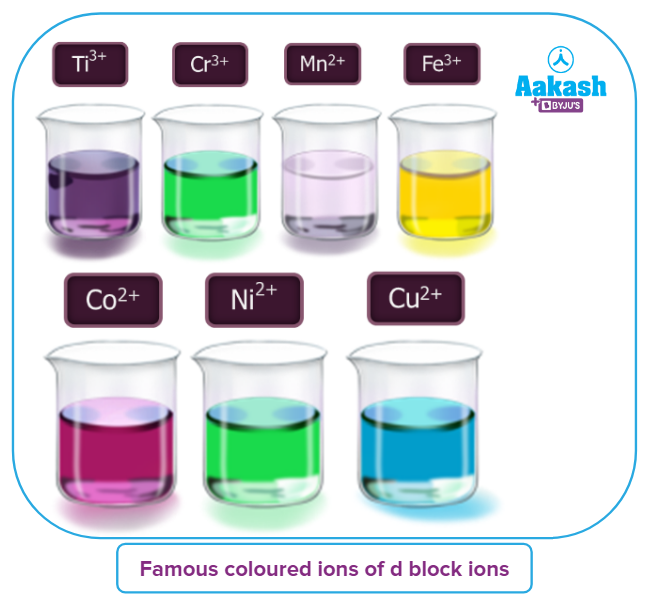
Some famous coloured ions of the 3d series that we have to study are mentioned below:
Ti+3: Purple
Cr+3: Green
Mn2+: Light pink
Fe3+: Yellow
Co2+: Pink
Ni2+: Green
Cu2+: Blue
In detail, We will study more in the chapter coordination compounds.
Application of d block elements:
- Catalytic characteristics of some transition metals are highly beneficial in the industrial synthesis of various compounds.
- Because transition metal ions are able to absorb visible light and migrate to higher energy orbitals, their compounds have vibrant colours.
- Iron is a transition metal that is widely utilised in construction and manufacturing.
- Titanium is utilised in aeroplane construction, artificial hip replacements, and nuclear power plant pipework.
- Stainless steel is produced with nickel.
- Electrical wiring is made of copper.
- Alloy formation takes place by using various d block elements like iron, nickel, and copper mixed with other elements.
Related videos:
https://www.youtube.com/watch?v=3XFtwsx4w6M (5:05-17:52) and (27:22-53:09)
Practice Problems
Q1. In the Haber process, which of the following metals serves as a catalyst?
A. Copper
B. Iron containing Mo.
C. Chromium
D. Nickel
Answer: B
Solution: The Haber Process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. The reaction is reversible and the production of ammonia is exothermic.In the presence of Mo, iron is the catalyst used, and it is not consumed during the reaction.
Q2. Which of the subsequent metal ions should exhibit colour?
A. Ti4+
B. Sc3+
C. Ti3+
D. Zn2+
Answer: C
Solution: Coloured transition metal ions have partially filled d orbitals, while colourless transition metal ions have fully filled (or empty) d orbitals. Ti4+,Sc3+and Zn2+have either fully filled or empty d orbitals so they don;t impart any colours. Ti3+has one unpaired electron and will impart purple colour
Q3.The magnetic moment value of which one of the following is 5.9?
A. Fe3+
B. Ni2+
C. Fe2+
D. Cu2+
Answer: A
Solution: The magnetic moment for 3d series can be given by the simple formula
For Fe3+, number of unpaired d electron are 5. Hence,
For Ni2+, number of unpaired d electrons are 0. Hence,
For Fe2+, number of unpaired d electrons are 4, Hence,
For Cu2+, number of unpaired d electrons are 3, Hence,
Hence, Fe3+ has magnetic moment value equal to 5.9
Q4. Transistion elements are easily converted into alloys due to their _______
A. Nearly same atomic size
B. Same atomic number
C. Same electronic configuration
D. None of the above
Answer: A
Solution: Atomic sizes of transition metals are remarkably comparable. To create a solid solution, one metal can simply remove the other metal from its lattice (alloy). In the molten form, transition metals can mix with one another. Alloy is created when two or more transition metals are combined in a molten state and cooled.
Frequently asked questions-FAQ
Q1. Why do we need transition elements?
Solution: Transition elements are crucial to life and evolution. Without iron, oxygen would not reach the brain, and life would be impossible. Without the Stone Age, the bronze, iron, and steel ages would never have occurred. Transition metals have become increasingly important as our population and economy have grown.
Q2.Who discovered the element cobalt for the first time?
Solution: The honour of the (re)discovery of cobalt in 1835 goes to the Swedish chemist Georg Brandt, whose country's scientists made significant contributions to the discovery of elements in the 18th century.
Q3.Why aren't all the elements in the d-block transition metals?
Solution: Although all d-block elements do not qualify as transition elements because they do not have fully filled d-orbitals, all transition metals are d-block elements. Zn, Hg and Cd are d block elements but are not transition elements.
Q4. Which element of the d-block is liquid at room temperature?
Solution: Mercury is the only liquid element in the d-block at standard room temperature.


