-
Call Now
1800-102-2727
Cyanide: Introduction, Nomenclature, Properties, Use, Toxicity, Practice Problems & Frequently Asked Questions
Is there anyone not familiar with the gold ornaments? A stupid question is it not? Everybody knows and loves and struggles to have as much as possible.
But, do you have any knowledge of how the gold is extracted and that the most deadly cyanide chemical is used to extract gold from mines?
Then what is cyanide and which properties make it so unique to gold extraction and also makes it deadly?
There are numerous applications for sodium cyanide (NaCN) in the mining sector. The primary application of cyanide is seen in the procedure used to extract gold and other precious metals from mines. Because sodium cyanide dissolves strongly in gold, it is specifically employed in gold mining. In the presence of oxygen and water, gold interacts with sodium cyanide to form sodium gold cyanide and sodium hydroxide.
Many commercially important chemical compounds, including cyanogen chloride, various nitriles, and cyanuric chloride, are produced with the aid of sodium cyanide. Cyanide is a potent nucleophile that contributes two electrons to the chemical bond that is formed during a reaction. Cyanide, a nucleophile, aids in the synthesis of nitriles in organic chemistry. Numerous compounds contain nitriles, which are widely used in the pharmaceutical sector.
Table of Contents:
- Introduction of Cyanide
- Nomenclature of Cyanide
- Properties of Cyanide
- Uses of Cyanide
- Toxicity of Cyanide
- Practice Problems
- Frequently Asked Questions(FAQs)
Introduction of Cyanide:
Inorganic compounds with the -CN group in its chemical formula is referred to as cyanide. A triple bond between a carbon atom and a nitrogen atom forms the cyano group. Cyanide compounds are found in a variety of sources.
Inorganic Cyanides:
In inorganic cyanides, the cyanide group is represented by the anion CN-. Both potassium cyanide and sodium cyanide are extremely lethal soluble salts.
Organic Cyanides:
The general term for organic cyanides is nitriles. In nitriles, a covalent bond connects the CN group to the carbon atom. For instance, the cyanide group is connected to methyl (CH3) in acetonitrile. Despite the facts that nitriles typically do not create cyanide ions, cyanohydrins do, making them much more dangerous.
Organic compounds containing -CN and also a higher priority group to -CN are given a prefix of cyano.
HCN:
Hydrocyanic acid also referred to as hydrogen cyanide is a highly flammable liquid that is extensively produced for industrial use. It is produced from acidified cyanide salts.
An extremely volatile liquid is hydrocyanic acid, often known as hydrogen cyanide (HCN). The Andrussow method, which combines ammonia, methane, and oxygen with a platinum metal catalyst, is used to create HCN. Acrylonitrile, which is required for the creation of synthetic rubber, acrylic fibres, and plastics, can be made using the HCN.
Cyanides from plant and microorganisms:
Fruit stones and some seeds contain sizable levels of cyanides as well. For instance, those of apricots, cyanide-laced bitter almonds, peaches, and apples. Cyanogenic chemicals are substances that break down chemically to release cyanide.
Plants typically protect themselves from herbivores by binding cyanides to sugar molecules in the form of cyanogenic glycosides. Cassava roots, commonly known as manioc, are a popular potato-like vegetable farmed in tropical regions and the raw material for tapioca. They contain cyanogenic glycosides.
Numerous fungi, algae, and bacteria also can create cyanide.
Nomenclature of Organic Cyanides:
A functional group is an atom or combination of atoms that determine an organic compound's characteristics and distinctive chemical reactions.
When identifying compounds with functional groups, a secondary suffix is added to the IUPAC name of the compound after the primary suffix to describe the type of functional group present in the organic compound.
The IUPAC Naming System's fundamental skeleton is
Secondary prefix + Primary prefix + Root word + Primary suffix + Secondary suffix
Secondary prefix - refers to the substituents attached to the parent chain
Primary prefix - refers to the nature of the parent chain (cyclo, bicyclo, spiro)
Root Word - refers the number of carbon atoms in the parent chain
Primary Suffix - refers the saturation of the parent chain
Secondary Suffix - refers the functional group
The parent chain is chosen to be the longest carbon atom chain that has the -CN group.
Along with the parent chain, the carbon atom from the -CN group is included.
As a result, the parent alkane is recognised, and its name is modified to include the suffix "nitrile." For instance, ethanenitrile is the term given to CH3CN in the IUPAC nomenclature.
Secondary prefix - cyano
Secondary suffix - nitrile
Special suffix - carbonitrile
|
Formula |
Common Name |
Secondary Prefix |
Primary Prefix |
Root Word |
Primary Suffix |
Secondary Suffix |
IUPAC name |
|
HCN |
Hydrogen Cyanide |
- |
- |
Meth |
ane |
nitrile |
Methane nitrile |
|
CH3CN |
Acetonitrile |
- |
- |
Eth |
ane |
nitrile |
Ethane nitrile |
|
CH3CH2CN |
Propionitrile |
- |
- |
Prop |
ane |
nitrile |
Propane nitrile |
|
CH3CH2CH2CN |
Butyronitrile |
- |
- |
But |
ane |
nitrile |
Butane nitrile |
|
CH3CH(CH3)CN |
Isobutyronitrile |
2-methyl |
- |
Prop |
ane |
nitrile |
2-methyl propanenitrile |
|
C6H5CN |
Benzonitrile |
- |
- |
Benzene |
Carbo |
nitrile |
Benzene carbonitrile |
Properties of Cyanide:
- Essentially, cyanide is a chemical with a white colour that can be found in granules or powder.
- It smells a lot like sour almonds if detected in its aqueous state.
- Cyanide has the chemical formula CN-, whereas hydrogen cyanide has the formula HCN.
- Hydrogen cyanide has a molecular weight of 27.0253 g mol-1 and a density of 0.6876 g cm-3.
- It has a 298.6 K boiling point and a 259.6 K melting point.
Uses of Cyanide:
- In clinical laboratories, sodium nitroprusside, a cyanide compound, is typically used to measure urine ketone bodies as part of a diabetic patient's follow-up. When people need an immediate drop in blood pressure, it is occasionally used as a vasodilator in vascular research and emergency situations. The cobalt in synthetic vitamin B12 contains a cyanide ligand due to the purifying process, and this ligand needs to be removed by the body before the vitamin molecule can be activated for biochemical use. During World War I, Japanese doctors temporarily used a copper cyanide chemical to treat leprosy and tuberculosis.
- Due to its ability to speed up the breakdown of these metals and their ores, cyanide is primarily utilised in the mining of Au (gold) and Ag (silver). The cyanide process involves mixing finely crushed high-grade ore with cyanide (at a ratio of roughly 1:500 parts sodium cyanide (NaCN) to ore); low-grade ores are piled up and sprayed with a cyanide solution (at a ratio of about 1:1000 parts sodium cyanide (NaCN) to ore).
- Aqueous cyanide hydrolyzes quickly, particularly when sunshine is present. Some heavy metals may get mobilised if Hg (mercury) is present. Arsenic replaces half of the sulphur atoms in the mineral known as arsenopyrite (FeAsS), which is comparable to fool’s gold (iron pyrite) . Arsenopyrite ores containing gold react similarly to inorganic cyanide. Cyanide is employed in electroplating to stabilise metal ions in the electrolyte solution prior to their deposition.
- In the vicinity of coral reefs, cyanides are illegally used to catch live fish for aquariums and seafood markets. Despite being contentious, dangerous, and damaging, the practice is nonetheless supported by the wealthy exotic fish industry.
- In New Zealand, possums, an introduced mammal that harms native species and transmits TB(Tuberculosis) among livestock, are among the pests that are controlled with cyanide. Possums can develop a fear of the bait, but this fear can be diminished by using cyanide pellets. There have been reports of native species, particularly the endangered kiwi, dying from cyanide poisoning.
- Metals are cleaned using sodium cyanide solution in manufacturing sectors. The chemical is employed in the dye industry to create colours. The chemical is utilised to create the electroplating solution in many different industries. In order to destroy pests that harm crops, farmers also employ agrochemicals and a pesticide.
- Additionally, cyanide is often used in various types of photography, such as sepia toning, and the manufacture of ornaments.
- Pesticides called cyanides are used to fumigate ships. Ant-killing cyanide salts have also been employed in the past as rat poison.
- Cyanide and cyanohydrins have been proven to enhance reproduction in a range of plant species, despite their reputation of being harmful.
Toxicity of Cyanide:
- A few cyanides are extremely toxic. The 4th complex of the electron transportation chain, cytochrome c oxidase, which is found in the inner membrane of eukaryotic mitochondria, is hindered by the cyanide anion. It interacts with the iron within this protein. When cyanide binds to this enzyme, it prevents cytochrome c from transferring electrons to oxygen. As a result, the electron transportation chain is disrupted, which prevents the cell from producing ATP for energy aerobically. The cardiac and nervous systems, which depend heavily on aerobic respiration, are the main areas impacted. It indicates histotoxic hypoxia.
- The most deadly substance is hydrogen cyanide, a gas that causes death when inhaled. One should always wear an external oxygen respirator supply when working with hydrogen cyanide for this reason.
Practice Problems:
Q1. Cyanide ion (CN-) acts as
(A) Ambident Electrophile
(B) Ambident Nucleophile
(C) Both A and B
(D) None of the above
Answer: (B)
Solution: Cyanide is an ambident nucleophile because it can interact either through carbon or nitrogen. Alkyl cyanides are created when cyanide ions attack carbon because the C-C bond is stronger than the C-N bond.
Q2. What is the following compound’s IUPAC name?
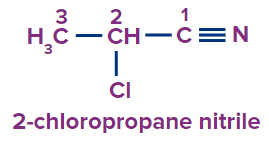
(A) 2-chloro propane nitrile
(B) 2-chloro Butane nitrile
(C) 2-chloro Pentane nitrile
(D) None of the above
Answer: (A)
Solution: The following compound has a cyanide functional group.
Root Word: The parent chain is of three carbon atoms. So, the root word will be prop.
Secondary Prefix: 2-Chloro
Primary suffix: ane
Secondary suffix: nitrile,
Hence the compound’s IUPAC name is 2-Chloro Propane nitrile.
Q3. What is the following compound’s IUPAC name?
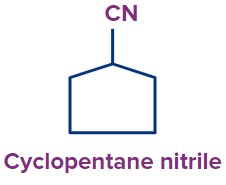
(A) Cyclo pentane nitrile
(B) Cyclo Butane nitrile
(C) Cyclo Hexane nitrile
(D) None of the above
Answer: (A)
Solution: The following compound has a cyanide functional group.
Root Word: The parent chain is of five carbon atoms. So, the root word will be pent.
Primary Prefix: Cyclo
Primary suffix: ane
Secondary suffix: nitrile,
Hence the compound’s IUPAC name is Cyclopentane nitrile.
Q4. What is the following compound’s IUPAC name?
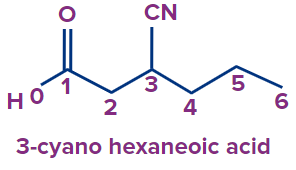
(A) 3-cyano hexanoic acid
(B) 2-cyano pentanoic acid
(C) 4-cyano hexanoic acid
(D) None of the above
Answer: (A)
Solution: The following compound has two functional groups: carboxylic acid and cyanide. As per the priority order carboxylic acid is a priority functional group.
Root word: The parent chain is of six carbon atoms. So, the root word will be hex.
Secondary Prefix: 3-cyano
Primary suffix: ane
Secondary suffix: -oic acid,
Hence the compound’s IUPAC name is 3-cyano hexanoic acid or 3-cyano hexan-1-oic acid.
Frequently Asked Questions (FAQs):
Q1. Why is it recognized that the cyanide group has an ambident nature?
Answer: Alkyl or aryl groups of carbon atoms can be joined to the cyanide group either through the carbon or the nitrogen atom. An ambident group is a particular kind of group that can be connected through two distinct sites.
Q2. Why does cyanide have a negative charge?
Answer: The carbon in the cyanide ion generates both a single pair of electrons and a fully negative charge. Although this does not completely eliminate confusion, the nitrogen atom also consists of a lone pair. The carbon end of the ion becomes the nucleophile when it is combined with a lone pair and a negative charge.
Q3. What effects will cyanide have on the human body?
Answer: Cyanide prevents the body's cells from using oxygen. The cells are perishing at this point. Since the heart and brain consume a lot of oxygen, cyanide is more hazardous to those tissues than it is to other tissues.
Q4. What is the cyanide antidote?
Answer: The kidneys can adequately eliminate them because cyanocobalamin is created when hydroxocobalamin and cyanide combine. The advantage of this strategy is that it stops methemoglobin from forming. The brand name Cyanokit is used to market this antidote package.
The antidote's goal was to create a substantial amount of ferric iron (Fe3+) to fight with cytochrome a3 for cyanide (so that cyanide will attach to it rather than the enzyme). Methemoglobin, which fights with cytochrome oxidase for the cyanide ion, is produced when nitrites oxidise haemoglobin.


