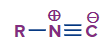-
Call Now
1800-102-2727
Curtius Rearrangement: Reaction, Mechanism & Application of Curtius Rearrangement Reaction, Practice Problems & FAQs
How do you arrange your books and notebooks on your shelf?
I guess we all love being organized about our books so that we can get easy access to any book whenever there is a requirement.
Imagine you have a well-maintained and organized bookshelf, where physics, chemistry, math and English books have separate columns.

Recently on your birthday, you received eight more chemistry books as a gift. But there is not enough space to accommodate all of them in the chemistry column.
What will you do in this scenario? Given you can’t create a new column.
Well, there are plenty of permutations and combinations through which you can rearrange them. Once you figure out what your requirements are, it will be easier to rearrange them in proper order.
Similarly in organic chemistry, whenever a compound is exposed to external factors or to another compound or metal, the respective compound may become unstable. Hence, to undo this instability, sometimes they rearrange their molecules. This mechanism is called a rearrangement reaction in organic chemistry.
Let’s understand one of the types of rearrangement reactions that is Curtius rearrangement and their mechanisms!
Table of Contents
- Rearrangement Reaction:
- Curtius Rearrangement:
- Mechanism of Curtius Rearrangement:
- Application of Curtius Rearrangement:
- Practice Problems:
- Frequently Asked Questions (FAQs):
Rearrangement Reaction:
Two distinct categories of organic chemical processes are referred to as "rearrangements." In a relatively short-lived intermediate, a rearrangement may involve the one-step migration of a H atom or a bigger molecular fragment.
The migration of a H atom or a bigger molecule fragment, however, may be one of the phases in a multi-step reaction known as a rearrangement.
The migratory group frequently attaches to an atom that was one of the direct neighbors of the atom to which it was initially linked in rearrangements. These types of rearrangements are known as [1,2] – rearrangements or [1,2] – shifts. The numerals 1 and 2 designate the subclass to which these rearrangements belong, and they can be thought of as sigma-tropic processes.
Curtius Rearrangement:
In 1891 Tiemann first suggested univalent short-lived species as intermediates in the Lossen rearrangement, later on by Stieglitz in 1896 in the Curtius rearrangement, and were also adopted by Curtius to explain various reactions of azides.
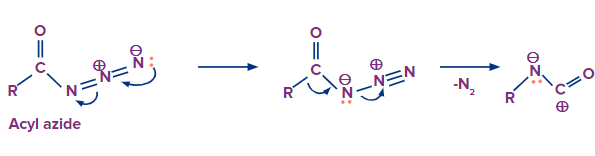
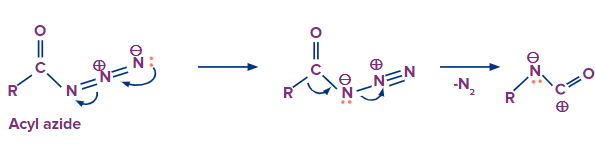
In the Curtius rearrangement, an acyl azide is pyrolyzed, releasing molecular nitrogen while also undergoing a rearrangement to become an isocyanate. Azides can disintegrate explosively, therefore they should be handled carefully. The isocyanate can be separated by carrying out the reaction in an aprotic solvent.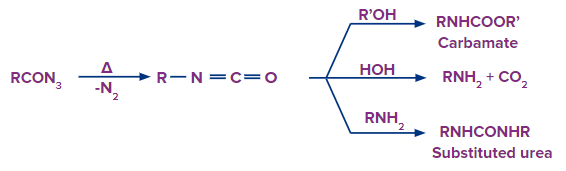
Mechanism of Curtius Rearrangement:
The mechanism of Curtius reaction follows two steps:
- Formation of intermediate
- Transformation of intermediate to final product
Step-1: Formation of intermediate
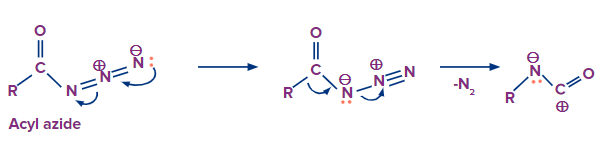
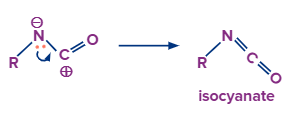
Nitrogen gas is released from the compound and the carbon atom starts moving towards the leaving nitrogen atom to occupy its position. A carbocation is produced as a result of this.
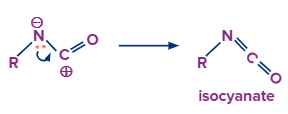
In this step the lone pair of electrons on the nitrogen atom will eagerly move towards the carbocation which results in the formation of isocyanate.
That’s how the intermediate is formed in this reaction.
Step-2: Transformation of intermediate to final product
If you have been to any restaurant then you know that the taste of gravy is similar to paneer butter masala and butter chicken. Because most of the restaurants prepare one gravy and add respective veggies to make a new product. Similarly, The beauty of this reaction is the resultant intermediate can be converted into 3 different products by changing the reagent.
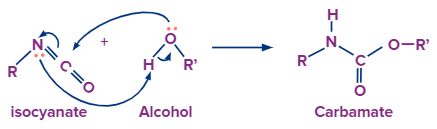
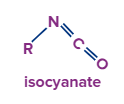
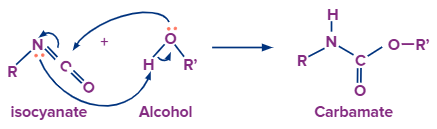
With Alcohol:
After the formation of isocyanate the nitrogen atom still has one lone pair electron which is donated to the hydrogen atom of the alcohol group. Now the disturbing electron density on the oxygen atom forces it to leave with the adjacent alkyl group and attach with the carbon atom to form carbamate.
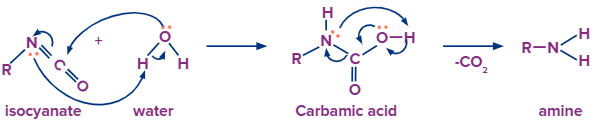
With Water:
In water, an oxygen atom is bonded to two hydrogen atoms unlike alcohol. When a water molecule is added to isocyanate. The O-H group with lone of electron on oxygen atom gets attached to the carbon atom of the intermediate resulting in carbamic acid. But as you can see from the reaction the product is very unstable due to higher electron density and it immediately releases carbon dioxide gas which forms amines.
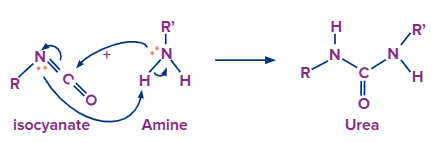
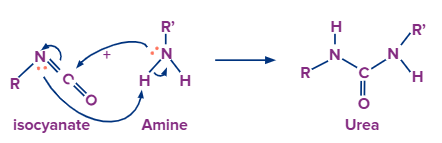
With Amine:
As amine has one nitrogen atom with a lone pair of electrons, hence we can also try a reaction with the resultant intermediate with amine. When amine is added to isocyanate it forms urea.
Application of Curtius Rearrangement:
There are various applications for the Curtius rearrangement in medicinal chemistry and drug discovery. Due to its tolerance for a wide range of functional groups and complete conservation of stereochemistry during the rearrangement, the Curtius rearrangement has been employed in the synthesis of a wide variety of pharmaceutical compounds with amines and amine-derived functional groups, such as ureas and urethanes. The Curtius rearrangement is used to create the medications tranylcypromine, candesartan, bromadol, terguride, benzydamine, gabapentin, igmesine, and tecadenoson.
Practice Problems:
Q1. Which of the following gas releases from acyl azide to form the corresponding carbocation?
- CO2
- N2
- H2
- O2
Answer: (B)
Solution: Nitrogen gas is released from the compound and the carbon atom starts moving towards the leaving nitrogen atom to occupy its position. A carbocation is produced as a result of this.
The step involves the following reaction
Q2. Which of the following intermediate is formed during the Curtius rearrangement reaction?
- Isocyanate
- Amide
- Cumene
- Imide
Answer: (A)
Solution:
Curtius rearrangement is a two step process in which intermediate is formed from acyl azide.
Step1:
Nitrogen gas is released from the compound and the carbon atom starts moving towards the leaving nitrogen atom to occupy its position. A carbocation is produced as a result of this.
Step2:
In this step the lone pair of electrons on the nitrogen atom will eagerly move towards the carbocation which results in the formation of isocyanate.
That’s how the intermediate is formed in this reaction.
Hence, the correct option is A
Q3. Which of the following products will form if the intermediate of curtius rearrangement reacts with alcohol?
- Carbamic acid
- Amine
- Carbamate
- All of the above
Answer: (C)
Solution:
The intermediate obtained from the Curtius rearrangement reaction is isocyanate.
After the formation of isocyanate, the nitrogen atom still has one lone pair electron which is donated to the hydrogen atom of the alcohol group. Now the disturbing electron density on the oxygen atom forces it to leave with the adjacent alkyl group and attach with the carbon atom to form carbamate.
Hence, the correct option is C.
Q4. Which of the following compounds can be used to obtain urea from acyl azide?
- Water
- Ethanol
- Aldehyde
- Amine
Answer: (D)
Solution:
When acyl azide undergoes a rearrangement reaction it forms an intermediate called isocyanate. When amine is added to isocyanate it forms urea.
Frequently Asked Question- FAQs:
1. Why do only carbocations show rearrangement reactions, and why not free radicals or carbanions?
Answer: Such processes would require higher energy transition states since thermal 1,2 shifts with retention at the migrating centre are prohibited in carbanions by orbital symmetry and a 1,2 shift with inversion at the migrating centre is geometrically challenging. Due to the higher energy, thermal 1,2 shifts involving carbocations are more frequent than the orbital symmetry permitted 1,2 shifts in carbanion systems. Between these two scenarios, a radical process might be realistically anticipated, and once more the transition state would be more energetic (because orbital symmetry is not permitted), making such rearrangements less frequent.
Although less frequent than carbocation shifts, radical and carbanion shifts do happen. High temperatures or weak bonds are necessary in the case of radicals. When the carbanion is stabilized, carbanionic rearrangements are more frequent. This is possible by situating the anion on a sp2or sp hybridized carbon rather than an sp3 hybridized carbon, or by attaching an electronegative heteroatom to the carbanionic centre.
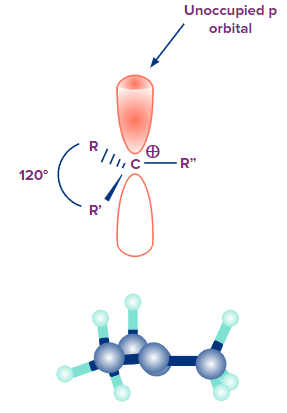
2. What is the geometry of carbocation?
Answer: The carbon atom becomes sp2 hybridized when a carbocation has three substituents, which results in a trigonal planar shape for the entire molecule. Generally, the bond angle between the substituents of the carbocation is 120°, and they are all located in the same plane. It only has six valence electrons, which are required to create three sigma covalent bonds with the substituents, hence the carbon atom in the carbocation is electron deficient. The unoccupied p orbital of the carbocation carbon is parallel to the plane made by the substituents. Carbocations make excellent Lewis acids because the p orbital can readily receive electron pairs during reactions.
3. What do you understand about azides?
Answer: Any chemical compound in the class of azides that has three nitrogen atoms arranged in a group is symbolized by the symbol (-N3). Azides are thought to be derivatives of hydrazoic acid (HN3), an inorganic salt like sodium azide (NaN3), or an organic derivative in which the hydrogen atom of hydrazoic acid is replaced by a hydrocarbon group, such as in alkyl or aryl azide (RN3), or by an acyl (carboxylic acid) group, such as in acyl azide.
4. What is isocyanate?
Answer: An organic compound with the functional group N-C+ is known as an isocyanide, also known as isonitrile or carbylamine. Its prefix is isocyano because it is an isomer of the closely related nitrile (-C-N).