-
Call Now
1800-102-2727
Crystal Defect: Definition of Defects, Stoichiometric, Non-stoichiometric and Impurity Defect, Practice Problems and FAQs
Have you ever seen or better participated in march past during republic day or independence day?
Well, we all love participating in that, wearing uniforms and going forward with unity.
You must have observed that in order to maintain the coordination of a group participating in the march, all members should align their shoulders and leg with everyone.
If that doesn’t happen then the rhythm will fall out. Sometimes it occurs due to one individual or sometimes due to a group of individuals, which becomes quite obvious for onlookers.
Crystal is solids that exhibit a long-range order in their arrangements. But, in crystals, due to several factors defects occurs. Let’s understand the different types of defects observed in crystals and their impact.
Table of Content
- Definition of Defects
- Line Defect
- Point defect
- Stoichiometric Defects
- Non-Stoichiometric Defects
- Impurity Defect
- Practice Problems
- Frequently Asked Questions (FAQs)
Definition of Defects
Imperfection or defect refers to any variation from the perfectly ordered arrangement of a crystal's component particles.
There are two types of defects
- Point defect
- Line defect
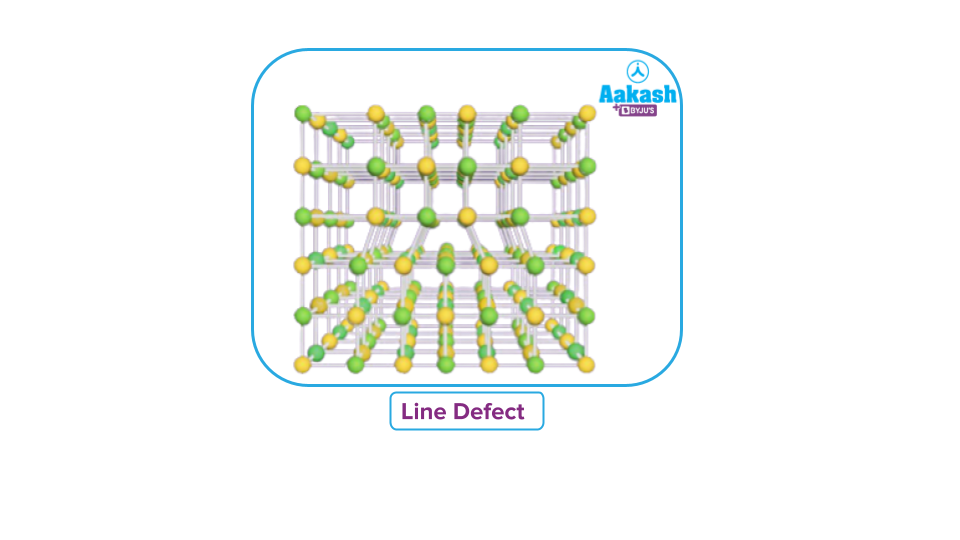
Line Defect
When the divergence from the ideal arrangement affects the entire row (or in one direction/axis) of lattice points, the defect is referred to as a Line defect.
In the final three rows of the crystal in the illustration, a different group (vertical line) of particles may be seen.
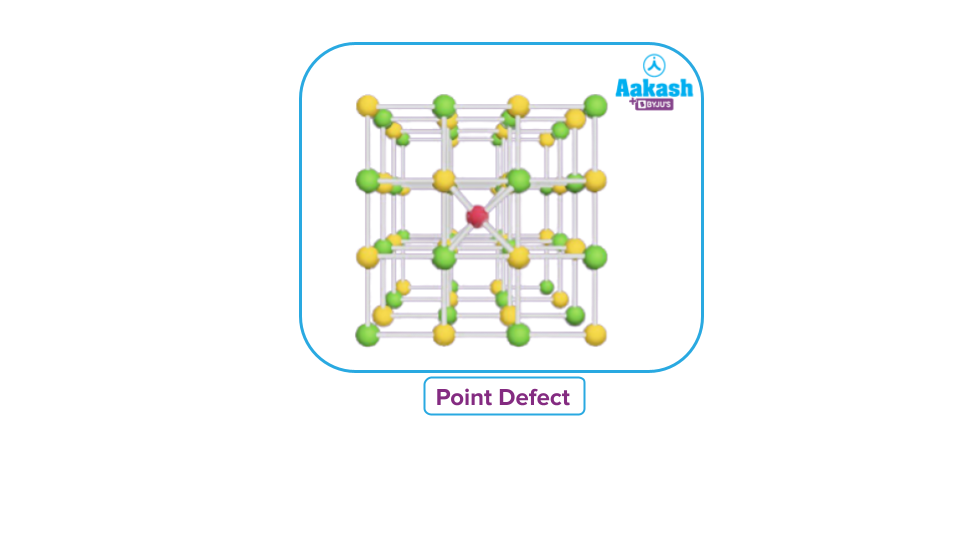
Point defect
A point defect occurs when there are differences or inconsistencies from the ideal arrangement around a point or an atom in a crystalline solid.
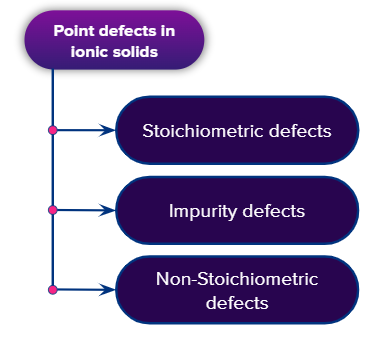
Point defects in a crystal may be classified into the following three categories
- Stoichiometric defects
- Impurity defects
- Non-stoichiometric defects
Stoichiometric Defects
Stoichiometric defects are those present in crystals that do not change the solid's stoichiometry, meaning that the ratio of cations to anions remains the same as indicated by the molecular formula.
These are also called intrinsic defects or thermodynamic defects and it is further classified into the following types:
- Defects shown by non-ionic solids
- Defects shown by ionic solids
Defects shown by Non-Ionic Solids
|
Vacancy defect |
Interstitial defect |
|
A crystal is said to have a vacancy defect or a thermodynamic defect when some of the lattice sites in it are vacant (as this defect arises due to absorption of heat from the surrounding). |
When additional constituent particles are found in the interstitial locations, the crystal is said to have an interstitial defect. |
|
As a result of the crystal's decreasing mass in the same volume, the substance's density lowers. |
Because of this defect, the substance's density increases because mass grows while the volume stays the same. |
|
|
|
Defects shown by Ionic Solids
|
Schottky defect |
Frenkel defect |
|
A Schottky defect is when an equal amount of cations and anions are missing from the lattice sites of an ionic crystal of type A+B in order to maintain electrical neutrality. |
If an ion is missing from its lattice site but occupies an interstitial site, electrical neutrality, as well as the stoichiometry of the compound, are maintained. This type of defect is called frenkel defect. |
|
Ionic compounds showing this type of defect have
|
This type of defect is shown by ionic solids with
|
|
For instance, NaCl, KCl, and CsCl |
Examples: silver halides (AgCl.AgI and ZnS) |
|
Due to this defect, the mass reduces while the volume stays constant when the number of ions decreases. As a result, the solid loses density, producing a defect resembling a vacancy. |
The density of the solid remains constant as there are no ions missing from the crystal. |
|
|
|
AgBr shows both Frenkel and Schottky defect.
Consequences of Schottky and Frenkel Defects
- As an ion moves from its lattice site to occupy a hole, it creates a new hole. A hole is created in the crystal, which causes the charge to move in the opposite direction. As a result, solids with these defects conduct electricity.
- Due to the presence of holes, lattice energy or stability of the crystal decreases.
- Dielectric constant of the crystal increases in case of Frenkel defects because in this defect similar charges come closer.
Non-Stoichiometric Defects
These are the imperfections in the crystal leading to a difference in, the ratio of the cations to the anions than expected from an ideal pure crystal lattice. The stochiometry of these crystals are hence different from the pure crystals and so are allied non-stoichiometric defects.
Nonstoichiometric defects may arise by
- Metal excess
- Metal deficiency
- Metal Excess Defect:
Metal-nonmetal ratio may become larger under two circumstances, One is due to the preferential loss of anions or by the excess addition of cations.
|
By anion vacancy |
By the presence of extra cations, |
|
A hole is created due to the missing of an anion. Now the hole is occupied by an electron to maintain electrical neutrality. The sites containing the electron thus trapped in the anion vacancies are F-centres. They are responsible for imparting colour to the crystal. |
Cations more than the stoichiometric amounts and present in the interstitial crystal sites result in a metal excess defect. The additional cation charges will be nullified by the presence of additional electrons in some other interstitial sites of the crystal structure. |
|
Example: When heated with sodium vapour, NaCl, deposition of excess of Na atoms gets deposited on the surface of NaCl the crystal. Diffusion of Cl- ions occurs to the surface where they combine with Na atoms who ionize by losing electrons Energy is adsorbed and a complimentary yellow colour is released by these electrons. |
Example: On heating, oxygen is released from ZnO giving a yellow colour to the crystal. Zinc ions and the electrons occupy the interstitial sites and thus making the crystal still neutral |
|
In crystals with Schottky defects, this defect can be found. |
In crystals with Schottky defects, this defect can be found. |
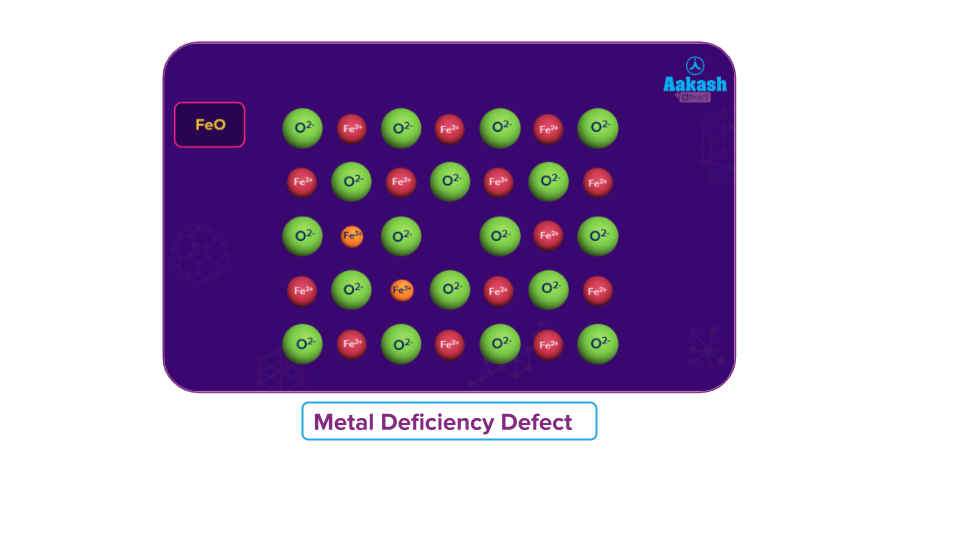
- Metal Deficiency Defect
This defect occurs when the metal shows variable valency. This defect usually occurs due to the missing of a cation having a higher charge in the adjacent lattice site.
Examples: FeO, FeS and NiO. Due to metal deficiency defects, the compounds are non-stoichiometric.
- FeO is found, mostly, with a composition of Fe0.95O, loss of some Fe2+ions is compensated by presence of a required number of Fe3+ions
Impurity Defect
An impurity defect is a crystal lattice distortion caused by an impurity (foreign atom/ion) occupying an interstitial site in the lattice or replacing the parent atom/ion in the regular sites.
Impurity defects can be classified into the following type is:
- Substitutional impurity
- Interstitial impurity
Substitutional Impurity
Defects in ionic crystals, can be introduced by adding impurities. Similar-sized cation substitute the existing cation of an ionic crystal.
Example:
When molten NaCl is crystallized having a small amount of SrCl2, some Na+ ion’lattice points are replaced with Sr2+ ions. Each divalent Sr2+ ion empties two Na+ sites. One such empty site will be occupied by strontium ion leaving the other site vacant.


The number of cationic vacancies generated = Number of Sr2+in the crystal
Other examples: solid solution of CdCl2 and AgCl.
Interstitial Impurity Defect
Here, some small foreign atoms (like B, C, N, H) are trapped in interstitial voids of the lattice without any chemical reaction.
Doping is the addition of impurities to a crystalline substance in order to alter its properties.
1. Introducing impurity defect in ionic solids
- Presence of ions other than that of the crystal ions will create impurity defect in ionic solids. If the other ions are of different valency from the crystal ions, then they will create vacancies related to the difference in valency in the crystal
- If a molten NaCl, containing a little SrCl2 as impurity is allowed to cool, in the crystals of NaCl formed, at some lattice sites, Na+ ions are substituted by Sr2+ ion. For every Sr2+ion thus introduced, two Na+ions are removed to maintain electrical neutrality. One of these lattice sites is occupied by Sr2+ and remains vacant. These vacancies will increase the electrical conductivity of the solid. Similar defects and behaviour are observed when CdCl2 is added to AgCl.
2. Introducing impurity defect in covalent solids
In the case of covalent solids such as silicon germanium (group 14 elements) which have 4 valence electrons in their outermost shell, the impurities added may be of the elements which may have more than 4 valence electrons (for example Group 15 elements like P or As which have five valence electrons) or of the elements which have less than 4 valence electrons (for example Group 13 elements like Ga which have three valence electrons).
Thus, the impurities added may be electron-rich or deficit. The defects thus introduced in the crystals are called electronic defects.
a) Doping with electron-rich impurities-
- Group 14 elements like silicon or germanium have four electrons in the outermost orbit. Hence, it normally forms four covalent bonds with the neighbouring atom.
- Group 14 elements have four electrons in their outermost orbit. Consider, that a group 14 element in a crystal structure is replaced by any of the elements of the 15 group which have five electrons in their outermost orbit. Group 15 element will use only four of its electrons for bonding and the fifth electron is completely free to move and conduct electricity of the group14 elements of silicon and germanium.
- As the increase in conductivity is due to negatively charged electrons, the silicon or germanium crystals doped with higher electron-containing elemental impurities are known as n-type conductors.
b) Doping with electron deficit impurities-
- When group 14 element like Si or Ge is doped with group 13 elements like B, Al, or Ga, the Si or Ge atom at some lattice are substituted by those of B, Al, or Ga.
- Now, as Group 13 elements have only 3 valence electrons, they can form three covalent bonds with the neighbouring silicon atoms.
- Thus, a hole is created at the site where the fourth electron is lost. The vacancy is known as electron vacancy or a hole
- An electron with a neighbouring atom jump to fill up this electron's hole but then an electron hole is created at the site from where electrons jumped.
- As this jumping continues, electrons and holes will be moving in opposite directions
- Now, when an electric field is applied, the electrons move towards the positively charged plate and electron holes will move towards the cathode like a cation. Hence, silicon and germanium doped with electron-deficit impurities are called p-type semiconductors.
Practice Problems
Q1. ZnS has a theoretical density of d gcm-3. If the crystal has a 4% Frenkel defect, then the actual density of ZnS should be:
A. d gcm-3
B. 0.04d gcm-3
C. 0.96d gcm-3
C. 1.0 d gcm-3
Answer: A)
Solution: The theoretical density of ZnS is d gcm-3. If the crystal has a 4% Frenkel defect,
then the actual density of ZnS does not change because cations missing from
their lattice sites occupies the interstitial sites. Therefore, it should be d
Q2. Silver bromide AgBr(s) crystals exhibit which of the following point defect and why?
A. Schottky defect
B. Frankel defect
C. Metal excess defect
D. Metal deficiency defect
- i & ii
- iii & iv
- i & iii
- ii & iv
Answer: A)
Solution: Schottky defects occur when ions are missing from their lattice point, while Frenkel defects occur when missing ions occupy interstitial sites. In the case of AgBr, both silver and bromide ions have almost similar radii with a radius ratio that is neither too large nor too close, So it exhibits both Frenkel and Schottky defects. The Frenkel defect is the most common defect in AgBr. It has a ccp lattice, in which Br atoms coexist with Ag atoms in all octahedral holes. When Ag transitions from octahedral to tetrahedral sites, only cations precipitate.
Q3. When a solid is heated, what kind of defect can occur? Which physical attribute is altered, and how?
Answer: When a solid is heated, the Crystal develops a vacancy defect. Because some atoms and ions completely leave the lattice site when heated, some lattice sides become vacant. The density of the substance decreases as some atoms/ions completely leave the Crystal as a result of this defect.
Q4. Which of the following is not a point defect
A. Stoichiometric defect
B. Non-stoichiometric defect
C. Impurity defect
D. None of the above.
Answer: (D)
Solution: defects in crystals have been classified into two categories
- Point defect
- Line defect
Point defect is further classified into 3 categories that is
- Stoichiometric defect
- Non-stoichiometric defect
- Impurity defect
Frequently Asked Questions (FAQs)
Q1. Why is there no Frankel defect in pure alkali metal halides?
Answer: Due to the large size of alkali metal ions it would be difficult to fit the ions in the interstitial sites of crystals. Hence, alkali metal halides don’t show a Frankel defect.
Q2. Why Stoichiometric defects are also called intrinsic defects?
Answer: Stoichiometric defects are so called because they do not alter the stoichiometry of the Crystal. they are called intrinsic defects because it is due to the deviation from the regular arrangement of atoms or ions within the Crystal and no external substance is added.
Q3. Is it possible to make a perfect crystal i.e, with zero defects?
Answer: No it is almost impossible to make a perfect crystal. Because only at absolute zero temperature a crystal will be defectless and attending 0 K is not possible. So crystal will have some amount of defect.
Q4. What is the difference between the interstitial defect of ionic and nonionic solids?
Answer: In ionic solids, an interstitial defect is also called a Frankel defect, the ions present at the lattice point vacate it and move to the interstitial position. In the interstitial defect non-ionic solids , foreign material come and occupy the interstitial positions







