-
Call Now
1800-102-2727
Close Packing Types: Close Packing, Close packing in 2D-Square and Hexagonal Close Packing, Close Packing in 3D- Hexagonal, Cubic and Body Centred Close Packing, Practice Problems and FAQs:
Who packs your dresses in suitcase when you go for a trip away- yourself or your seniors?
Two suitcases, packed by two people are here. You can guess which was packed by whom?

The point to note is that the neatly packed suitcase holds much more dresses and in a neat manner for ready use after than the unorganized loosely filled suitcase.
The below image shows a certain number of balls stacked over the gaps. They are arranged in a way to minimize the empty spaces between the balls and maximum utility of the available space in the box to accommodate more number of balls in the box.

Table of content
- Close packing
- Square close packing
- Hexagonal close packing
- Close packing in 3-D
- Voids
- Cubic Close packing
- Body centred close packing
- Practice problems
- Frequently asked questions(FAQs)
Close packing
In crystalline solids, the constituent particles are close-packed, leaving very less vacant spaces. In measuring the efficiency of packing, the particles are assumed to be spheres of identical size.
Let us understand close packing in 1D, 2D, and 3D.
Close packing in 1-D
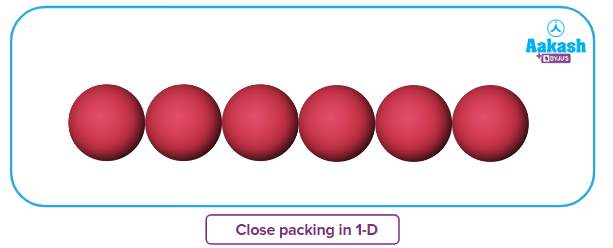
There is only one way of arranging spheres in a one-dimensional, close-packed structure, which is to arrange them in a row touching each other. Each sphere in this configuration is in contact with two of its neighbours. A particle's coordination number is the sum of its nearest neighbours' numbers. Thus, in one-dimensional close-packed arrangement, the coordination number is 2.
In a one-dimensional, tightly packed structure, there is only one way to arrange spheres, and that is to arrange them in a row touching one another. In this arrangement, each sphere has two neighbours with whom it can make contact. The coordination number of a particle is equal to the sum of the numbers of its closest neighbours. Therefore, the coordination number in a one-dimensional close-packed arrangement is two.
Close packing in 2-D
A two-dimensional, close-packed structure can be generated by placing rows of close-packed spheres next to one another,
By arranging rows of closely packed spheres next to each other, a two-dimensional structure can be created. say on a table. The arrangement of spheres is done by stacking the rows of closely packed spheres in two possible ways.
● Square close packing
● Hexagonal close-packing
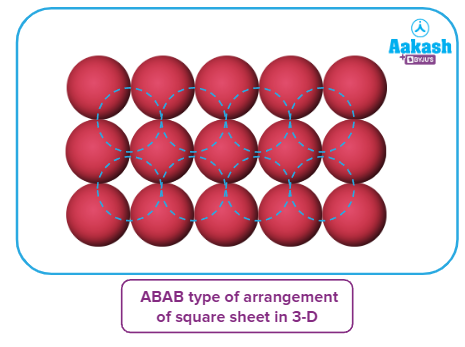
Square close packing
The spheres of the second row are placed in contact with the first one such that the spheres of the second row are exactly parallel to those of the first row. The spheres of the two rows are aligned along length and breadth of a square at right angle. If we call the first row an A-type row, then the second row, being exactly the same as the first one, is also of A type. Similarly, we can place more rows to obtain an AAA type of arrangement.
The second row's spheres are positioned in close proximity to the first row so that they are perfectly parallel to one another. The spheres of the two rows are at a right angle alignment along the length and width of a square. If the first row is an A-type row, then the second row is also an A type row because they are identical to one another. Similarly, we can add more rows to create an arrangement resembling AAA.
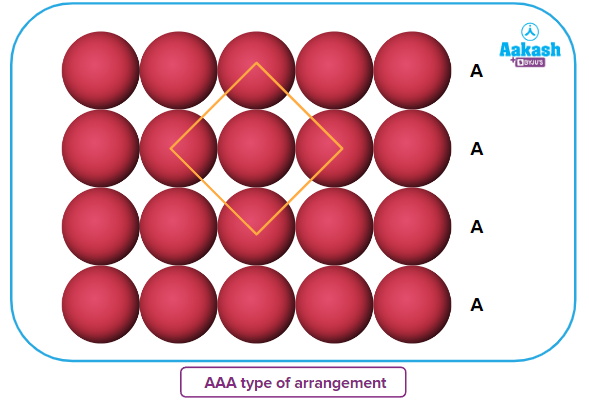
Each sphere in this configuration is in contact with four of its neighbours. As a result, the coordination number in two-dimensional square close packing is 4, and a square is created by joining the centres of these four immediately adjacent spheres. Hence, this packing is known as square close packing in two dimensions.
The arrangement here places four of each sphere's neighbours in contact with it. Because of this, the coordination number for two-dimensional square close packing is four, and a square is formed by connecting the centres of these four immediately adjacent spheres. The two-dimensional term for this packing is square close packing.
Hexagonal close packing
In this close packing, the second row is placed in the gap of the first row in a staggered manner such that its spheres fit in the depressions of the first row.
In this close packing, the second row is arranged in the opening left by the first row in a staggered fashion so that its spheres fit in the depressions of the first row.
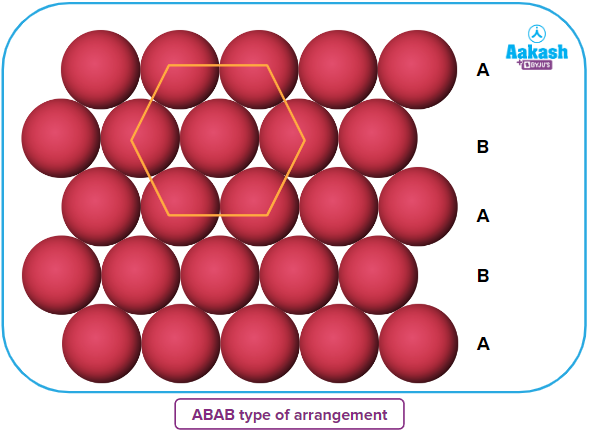
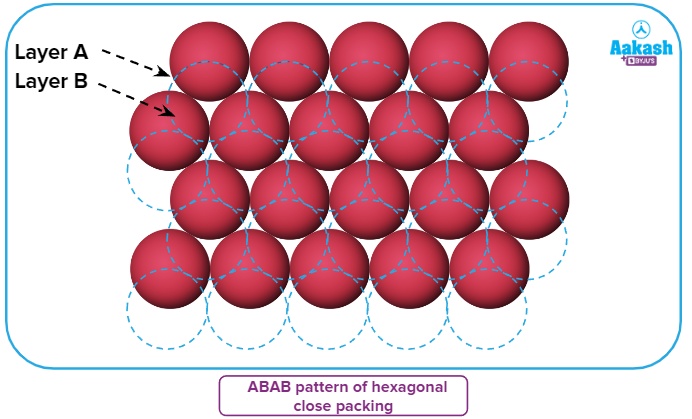
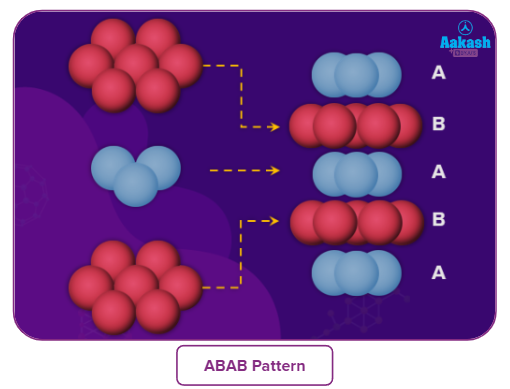
- If the arrangement of spheres in the first row is of A type, then the one in the second row is different and can be called B type. When the third row is placed adjacent to the second in a staggered manner, its spheres are aligned with those of the first layer. As a result, this layer is likewise an A layer. The spheres of the similarly placed fourth row will be aligned with those of the second row (B type). This arrangement is therefore of the ABAB type.
- If the first row of sphere arrangements falls under the category of A type, the second row's arrangement falls under the category of B type. The third row's spheres line up with those of the first layer when it is positioned next to the second row in a staggered manner. Consequently, this layer is also an A layer. The spheres of the fourth row, which is positioned similarly, will line up with those of the second row (B type). As a result, this arrangement is of the ABAB type.
- Each sphere is in contact with six of its neighbours, and the coordination number in two-dimensional hexagonal close packing is 6.
- The coordination number in a two-dimensional hexagonal close packing, where each sphere is in contact with six of its neighbours, is six.
- These six spheres' centres are located at each of the hexagon's four corners. This arrangement is hence referred to as two-dimensional hexagonal close packing.
- The centres of these six spheres are at the four corners of the hexagon. As a result, this configuration is known as two-dimensional hexagonal close packing.
- In hexagonal close packing, there is less free space and packing is more efficient than square close packing.
- In comparison to square close packing, hexagonal close packing requires less free space and packs more closely.
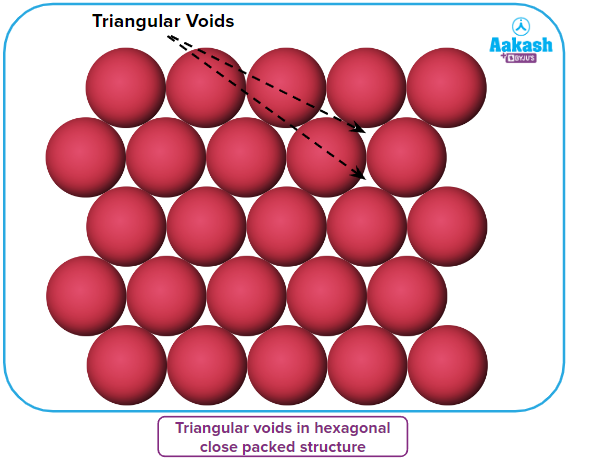
- It can be seen in below figure that the layers have some voids (empty spaces). They are triangular in shape. The triangular voids can be occupied not on the same side but from opposite sides giving rise to different 3D arrangements.
- The layers in the figure below can be seen to have some voids (empty spaces). They have a triangular form. The triangular voids can be filled from opposite sides rather than the same side, resulting in various 3D arrangements.
Close packing in 3-D
They can be obtained by stacking two-dimensional layers one above the other The different ways of forming 3D close packing are given as follows:
a) From 2D square close packing
b) From 2D hexagonal close packing
c) BCC close packing
From 2D square close packing
- We apply the same principle that was used when placing one row next to another when we stack the second square close-packed layer over the first. The second layer is positioned on top of the first layer so that its spheres are directly above its front counterparts.
- In this configuration, the spheres of the two layers are precisely vertically and horizontally aligned. Similarly, we can stack additional layers on top of one another.
From 2D Hexagonal close packing
A three-dimensional close-packed structure can be generated by placing 2D hexagonal close-packed layers one over the other.
By stacking 2D hexagonal close-packed layers on top of one another, a three-dimensional close-packed structure can be created.
- Take layer A, a two-dimensional hexagonally close-packed layer, and arrange layer B on top of it in a such that the spheres of layer B are positioned in the depressions of layer A.
- Layer B should be positioned on top of layer A, a two-dimensional layer with hexagonally close-packed hexagons, so that its spheres fit inside the dip of layer A
- Let's refer to the second layer as layer B as the spheres of the two layers are not perfectly aligned.
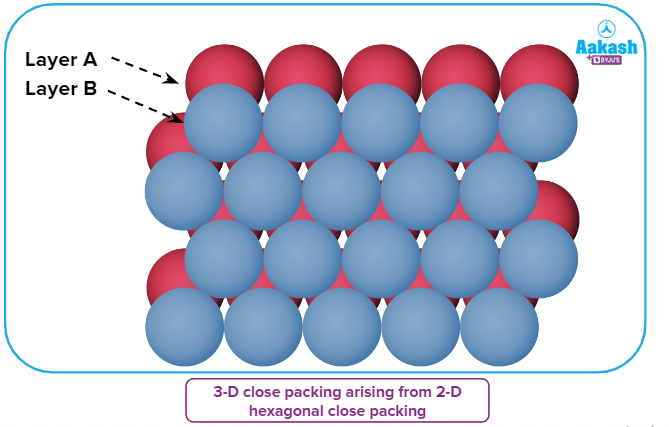
It can be observed that all the triangular voids of the first layer are not covered by the spheres of the second layer.
It is clear that the spheres of the second layer do not completely enclose all of the triangular voids of the first layer. Here, out of the six voids/depressions around each sphere (in one hexagonal unit cell) in the first layer A, only three are directly covered by placing spheres of the second layer B.
putting the third layer on top of the second
When the third layer is placed over the second layer, there are two possibilities, which are given as follows:
● Covering tetrahedral voids
● Covering octahedral voids
Covering tetrahedral voids
Tetrahedral voids of the second layer (blue) may be covered by the spheres of the third layer (yellow). In this case, the spheres of the third layer are exactly aligned with those of the first layer. As a result, the sphere pattern is repeated in alternate levels. ABAB... pattern is a common way to write this pattern. The acronym HCP stands for hexagonal tightly packed structure. Many metals, including magnesium and zinc, have this kind of atom arrangement.
The spheres of the third layer may cover the tetrahedral voids of the second layer (blue) (yellow). In this instance, the spheres from the third and first layers are perfectly lined up. The result is that alternate levels of the sphere pattern are repeated. This pattern is frequently written as the ABAB... pattern. Hexagonal closely packed structure is referred to by the abbreviation HCP. This type of atom arrangement can be found in many metals, such as Mg and Zn.
Voids
There are two types of voids, and they are as follows:
(i) Tetrahedral void
(ii) Octahedral void
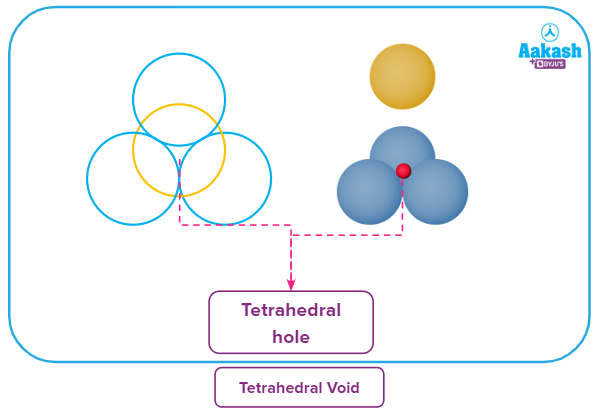
Tetrahedral voids
If the sphere of layer B (second layer) is placed over the triangular void of layer A (first layer), the type of void formed is known as a tetrahedral void. A sphere placed in the void will touch four spheres, three from the first layer and one from the second layer, forming a tetrahedral structure.
Octahedral void
Octahedral void
If the triangular void of layer B is placed above the triangular void of the layer A, the void formed is known as an octahedral void.
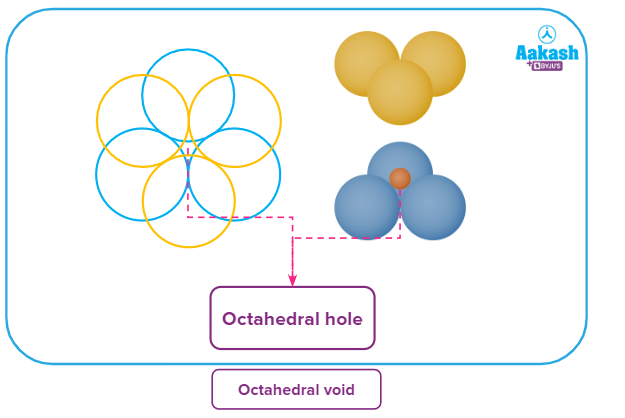
In this figure, we can see that triangular void formed by three blue spheres of one layer stacked above the triangular void formed by the three red spheres of another layer, forming an octahedral void.
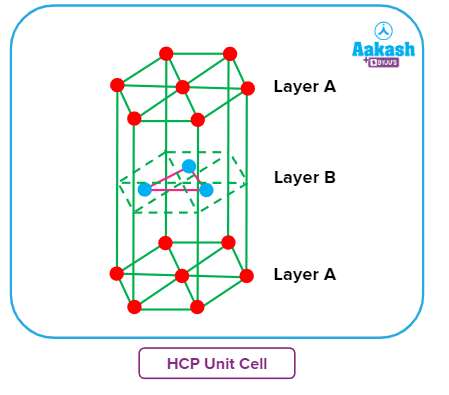
Unit cell in HCP arrangement:
A unit cell is obtained by finding the repeating units or arrangements which, on repeating in every direction, forms the HCP packing.
Drawing hexagons of the first layer and continuing it in every direction forms layer A. In the next layer B, the spheres forming a triangle (as shown by three blue spheres) are the repeating units.
The unit cell is then obtained by joining the hexagonal repeating units of the two A layers with the triangular repeating units of B layer in between them. The obtained unit cell is known as a hexagonal
close packing unit cell.
Coordination number of spheres in HCP:
- For a HCP unit cell, consider the middle yellow sphere consisting of the number of the nearest neighbouring particles, i.e., equal to 12, so that the coordination number is 12. Each sphere touches six spheres in its layer, three above it and three below it. So, its coordination number is also 12.
- Note that the coordination number will be the same, irrespective of the atom you take as a reference. Examples: Mg, Cd, Zn, Ti and Be
Cubic Close packing
Placing the third layer
- If the spheres of the third layer are placed over the second layer such that its spheres cover the octahedral voids, then the third layer will not align with either the first or the second layer, but the fourth layer above the third layer will repeat the orientation of first layer. This pattern of layers is known as an ABC–ABC pattern.
- Such a structure is known as cubic close packing (CCP) or face-centred cubic (FCC) packing.
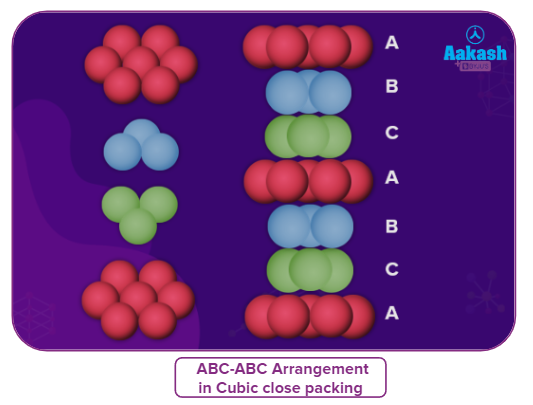
The completely different arrangement of red, yellow, and blue spheres one over the other forms an ABCABC pattern, the red spheres (A), followed by the blue spheres (B), and then the yellow spheres (C) from the bottom.
● The packing in Ca, Sr, Cu, Ag, Au, etc., follows this type of arrangement.
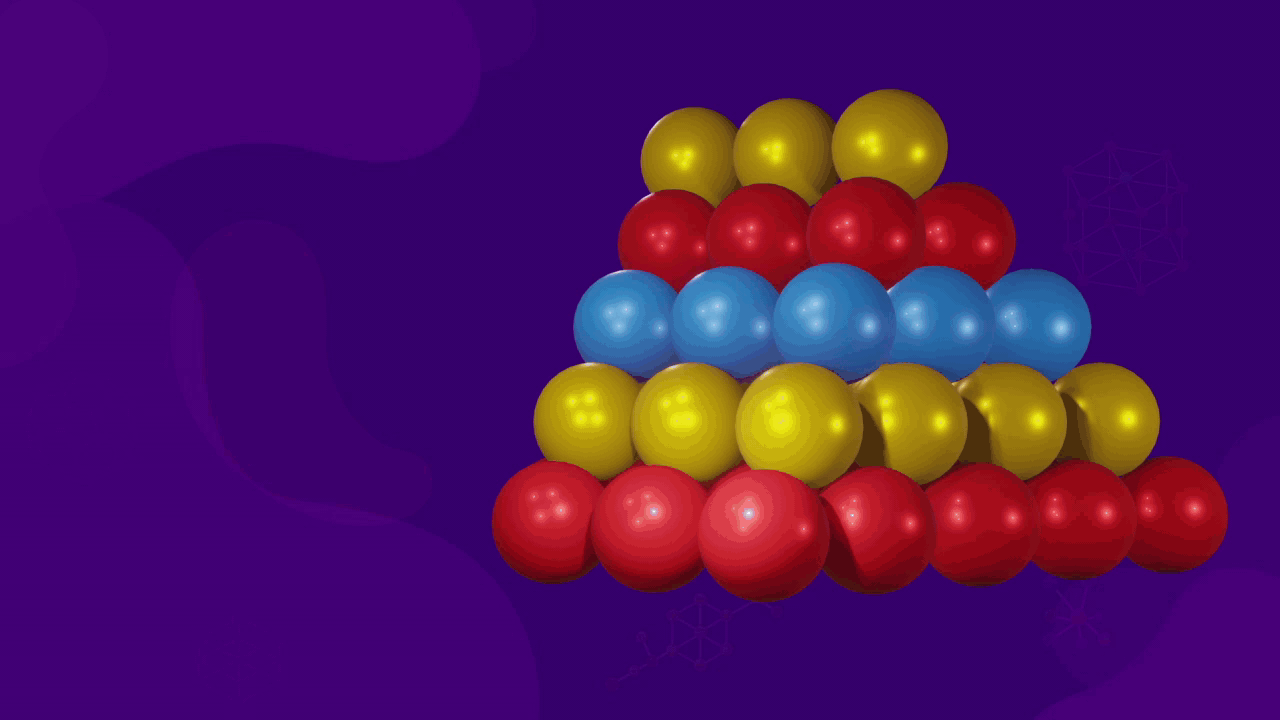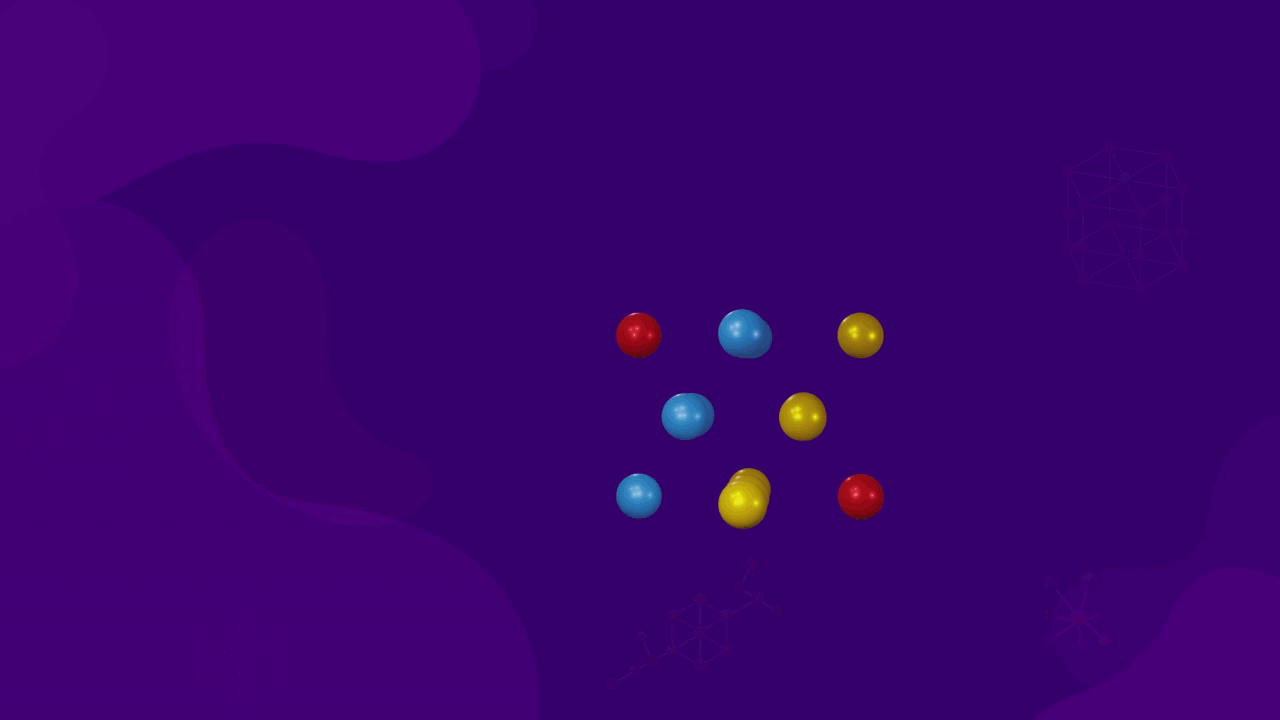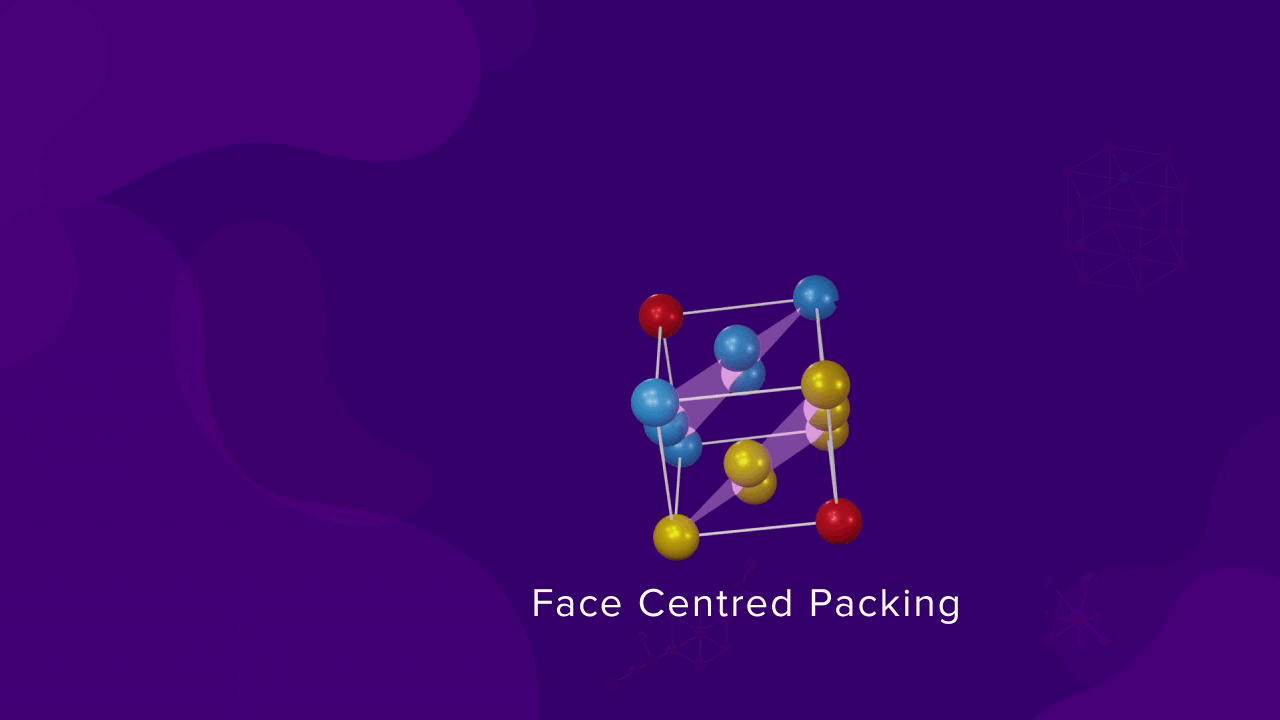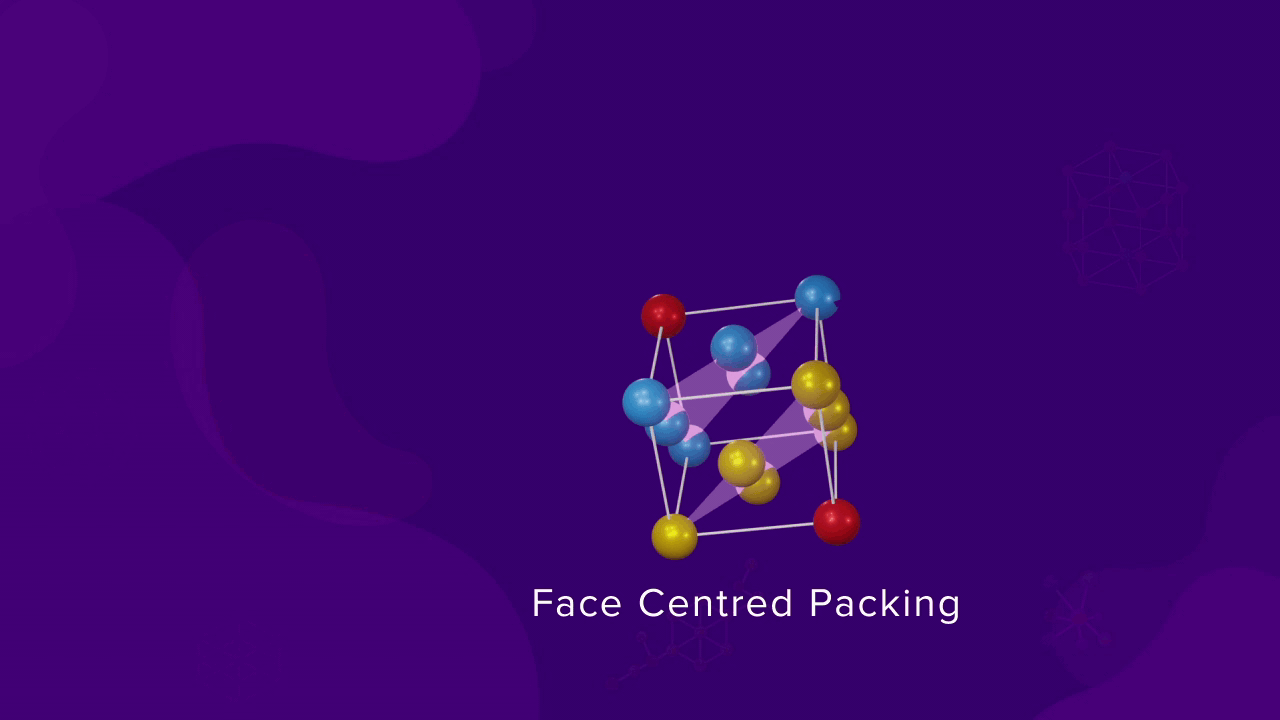
Unit cell in Cubic close packing
Let us consider that a sphere in the first layer (A) is in the corner and the spheres of layer B, which are above the sphere of layer A, are at the face centre of the faces of the unit cell and also at the corner. The other face centres are occupied by the spheres of layer C and even some corner are also occupied by layer C. Finally, the last corner is occupied by the sphere of another layer A, forming an FCC unit cell.
Coordination of particles in FCC
- For a CCP or FCC packing, as the number of nearest neighbours of a particle is 12, the coordination number is also 12. Each sphere touches six spheres in its layer, three above it and three below it.
![]()
- The corner sphere of an FCC unit cell is in touch with 12 face-centered spheres, which belong to the eight unit cell of which that corner is a part of.
- If we consider XY, YZ, and XZ planes passing through the corner atom, each plane contains four faces and each face has one face-centered sphere, which is in touch with the corner atom. Since there are a total of 12 faces and 12 face-centred spheres, the coordination number for FCC is also 12.
Body Centred Close packing (BCC)
- Consider the spheres in the first layer as square-packed in a slightly opened up manner, i.e., not touching each other.
- The spheres in the second layer are placed in the voids i.e., at the top of the hollow spaces in the first layer (not touching each other).
- The spheres in the third layer are placed above the voids of the second layer. They are exactly as the spheres in the first layer, and those of the fourth layer are exactly as the ones in the second layer, forming a pattern of ABAB type. Such a structure is known as BCC close packing.
- The unit cell formed is known as the body-centered cubic unit cell.
Practice problems
Q1. In which of the following states of matter constituent particles are absolutely closely packed?
A. Solid
B. Liquid
C. Gas
D. Plasma
Answer: (A)
Solution: In solid state, the constituent particles are closely packed and held together by strong force of attraction between them. The constituent particles only oscillates to their mean position. Hence, solids have a definite shape and volume.
The constituent particles of a solid are tightly packed and held together by a strong force of attraction. Only oscillation at the mean position is allowed for the constituent particles. Solids, therefore have a distinct shape and volume.
Q2. What will be the coordination number, if the particles are arranged in one dimensional close packing?
A. 1
B. 2
C. 3
D. 4
Answer: (B)
Solution: The constituent particles are arranged in a row in one dimensional close packing, each particles are in contact with two other particles. Hence, the coordination number in one dimensional close packing is 2.
Each constituent particle is in contact with two other particles and is arranged in a row in a one-dimensional close packing. Consequently, in one-dimensional close packing, the coordination number is two.
Q3. In how many ways, you can arrange two particles to get a two dimensional close packing?
A. 3
B. 1
C. 2
D. 5
Answer: There are two ways through which we can generate two dimensional close packing from one dimensional close packing
- If one dimensional close packing is placed with another one dimensional close packing in such a way that the particles stays just below the above particle.
- If one dimensional close packing is placed with another one dimensional packing in such a way that the particles stays below the depression of the above arrangement.
- Correct option is C.
We can create two-dimensional close packing from one-dimensional close packing in two different ways.
- If two one-dimensional close packings are arranged so that the lower particle remains immediately beneath the upper particle.
- If two one-dimensional close packings are arranged so that the particles remain below the dip created by the previous arrangement.
Hence, correct option is C
Q4. Voids in two-dimensional hexagonal close packed structure are ___________ in shape.
A. rectangular
B. triangular
C. hexagonal
D. circular
Answer: When one row of a one-dimensional structure is placed below another in such a way that the spheres of the second row fit into the depressions of the first row, generating triangular voids between them, a two-dimensional hexagonal close packed structure is generated.
A two-dimensional hexagonal close packed structure is created by stacking rows of a one-dimensional structure so that the spheres of the second row fit into the depressions of the first row, creating triangular voids between them.
Corect option is C.
Frequently asked questions(FAQs)
Q1. Which close packing arrangement is the most efficient one?
Answer: In both cubic and hexagonal closest packing, the arrangement efficiently takes up 74 percent of the space. The second layer of spheres is placed on half of the depressions of the previous layer, similar to hexagonal closest packing.
Q2. Are FCC and CCP similar?
Answer: Face Centered Cubic (FCC) and Cubic Close Packed (CCP) are two more names for the same lattice.
Q3. Which metal shows hexagonal close packing?
Answer: Titanium, zirconium, magnesium, and other hexagonal close-packed (HCP) metals and alloys are widely employed in a range of industrial industries.
Q4. What does "close packing" in solid structures mean?
Answer: The packing is done so that the constituent particles will leave the least amount of vacant space between them by utilizing the maximum amount of space that is available. Closed packing is the name for this style of packaging.


