-
Call Now
1800-102-2727
Classification of Hydrocarbons – Introduction, Types, Properties, Preparation, and Uses of Hydrocarbons
Every nation on earth depends heavily on the global economy to survive, and as a result, our survival is either directly or indirectly impacted by it.
Which industry, to put it plainly and simply, helps countries make money the most? Can you identify?
Well, it can be determined by looking at the list of the most popular imports from across the world, and the top purchasing countries reveal which goods are in high demand on the world market. The products that top the importing charts are crude oil and various petroleum products.

Oil and natural gas, the two primary fuel sources in the world, are important players in the energy sector and have an effect on the international economy. Processes and systems for the production and delivery of oil and gas are extremely complex, capital-intensive, and reliant on cutting-edge technology.
But in Chemistry, how does that even matter to us? Well, the response is really simple. Hydrocarbons are the products of oil and gas that are being discussed! In order to better grasp the state of the world, it is crucial that we study and learn about hydrocarbons and their categories, whether we are novice learners or seasoned experts.
In this article, we will get to know about hydrocarbons and their classification.
TABLE OF CONTENTS
- Introduction to Hydrocarbons
- Classification of Hydrocarbons
- Comparison between Unsaturated and Saturated Hydrocarbons
- Uses of Hydrocarbons
- Practice Problems
- Frequently Asked Questions – FAQ
Introduction to Hydrocarbons
Carbon and hydrogen atoms are the two main types of atoms that make up the organic molecules known as hydrocarbons. These gases are typically colourless and odourless. Hydrocarbons can have simple or complex structures, depending on their nature. Alkenes, alkanes, alkynes, and aromatics are the other four key categories into which they are usually divided.
Liquefied petroleum gas, or LPG, is a commercial fuel made from a number of hydrocarbons, including propane and butane. Another important aromatic hydrocarbon, benzene, is used as a starting point in the production of synthetic medicines. Carotene is a different hydrocarbon that is a common organic pigment in carrots.
Hydrocarbons have the molecular formula CxHy. Plants and trees are known to contain hydrocarbons. Carotenes, for example, are an organic pigment found in green leaves and carrots. Natural crude rubber is composed of 98 percent hydrocarbons. Furthermore, they have a lot of internal energy, which makes them important.
Classification of Hydrocarbons
Previously scientists used to categorise hydrocarbons as either aliphatic or aromatic. The hydrocarbons were categorised using the sources and properties. As a result, it was found that aromatic hydrocarbons included components created by the chemical breakdown of plant extracts, whereas aliphatic hydrocarbons were made by the chemical degradation of fats or oils. Today, however, we classify hydrocarbons according to their structure rather than their place of origin. The classification of hydrocarbons can be represented as follows:
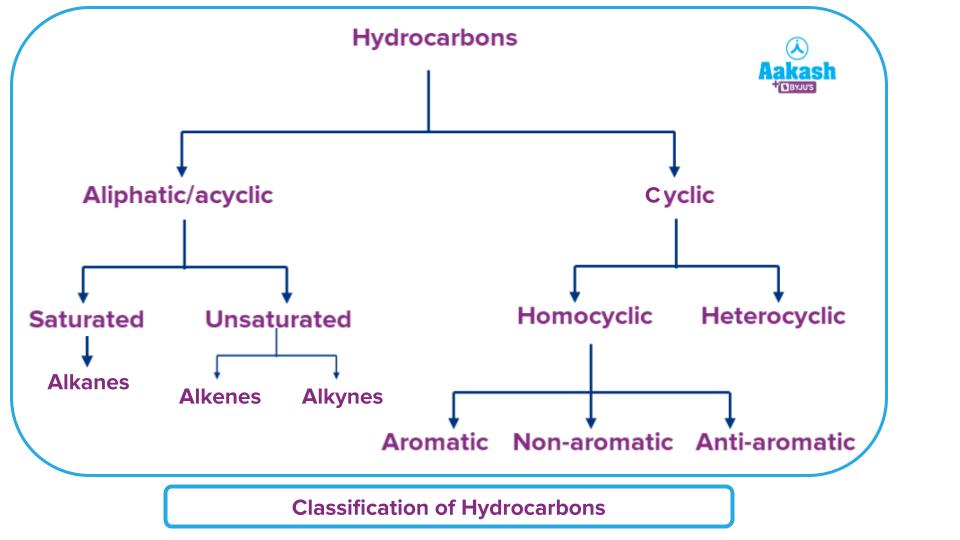
Aliphatic/Acyclic Hydrocarbons
The chain structure of aliphatic hydrocarbons are entirely straight and devoid of carbon rings. It is made up of chains of carbon and hydrogen atoms connected by single, triple, or double bonds. These chains can occasionally be found in non-aromatic materials. Aliphatic hydrocarbons are broadly classified into Saturated and unsaturated hydrocarbons. Alkanes, which are open chain hydrocarbons with a carbon-carbon single bond, make up the majority of saturated hydrocarbons. The bond often takes the shape of a covalent bond. Due to their inert nature, these substances do not easily. Let us look into the two larger subdivisions of aliphatic hydrocarbons, i.e., ‘saturated’ and ‘unsaturated’ hydrocarbons, in detail below.
Saturated Hydrocarbons
Saturated hydrocarbons are simply hydrocarbons with single carbon-carbon bonds throughout. Saturated hydrocarbons are hydrocarbons with a strong connection between each of the five carbon atoms. In saturated hydrocarbons, which are the simplest hydrocarbons, a single bond tightly holds the carbon-carbon and carbon-hydrogen atoms together. Both double and triple bonds are absent from such hydrocarbons. All carbons in these are sp3 hybridised.
Alkanes: Alkanes are basically saturated hydrocarbons with the simple formula CnH(2n+2). Alkanes are called paraffins. They are inert towards general reagents under normal conditions. Non-reactive as they are non-polar and have no π-bonds (σ-bonds only).
Examples: Methane CH4, ethane C2H6, etc.,
Unsaturated Hydrocarbons
Unsaturated hydrocarbons are made up of either a double bond or a triple bond between the two neighbouring carbon atoms. Unsaturated hydrocarbons are composed of carbon-carbon atoms bound together by double, or triple bonds.
Alkenes: Since an alkene only consists of a double bond between two carbon atoms, the simplest alkene will have two carbon atoms in each molecule. An alkene with just one carbon does not exist. With only two carbon atoms, ethene is the most basic alkene. Alkenes have a general formula of CnH2n.
Examples: Ethene H2C=CH2, propene CH3-CH=CH2, etc.,
Alkynes: Alkynes, which are compounds with carbon-carbon triple bonds (C≡C), are different from alkenes, which are compounds with carbon-carbon double bonds (C=C). Alkynes have the general formula of CnH(2n-2). Since an alkyne only includes a triple bond between two carbon atoms, the simplest alkyne will only contain two carbon atoms in its molecules. There is not a single-carbon alkyne in existence. With only two carbon atoms, ethyne is the most basic alkyne. Unsaturated hydrocarbons react more quickly than saturated hydrocarbons. Alkenes and alkynes so frequently have higher chemical reactivity than alkanes.
Examples: Ethyne HC≡CH, propyne CH3-C≡CH, etc,.
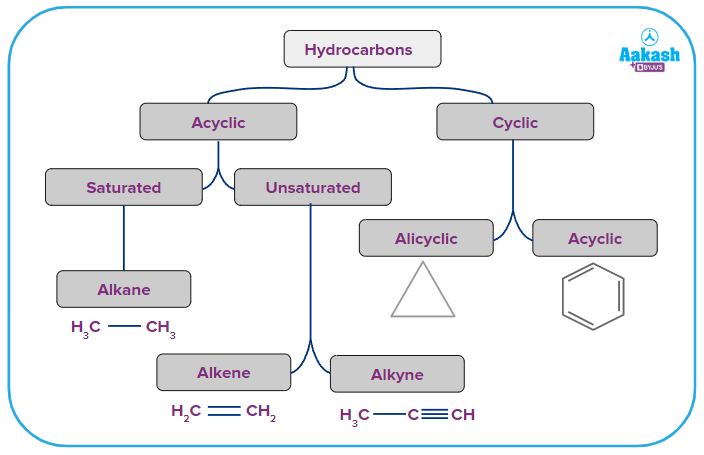
Cyclic Hydrocarbons
Cyclic hydrocarbons are composed of carbon and hydrogen atoms bonded to form ring structures. They are broadly classified into homocyclic and heterocyclic hydrocarbon compounds.
Homocyclic Compounds
Homocyclic compounds are also referred to as carbocyclic compounds. Carbocyclic chemicals, sometimes referred to as carbocycles, are homocyclic compounds. In homocyclic compounds, the ring members are made up of atoms from the same element as the rest of the complex. Homocyclic substances in organic chemistry exclusively include carbon atoms. There are four types of homocyclic molecules. Aromatic, non-aromatic, anti-aromatic and alicyclic or (cycloalkanes). Cycloalkanes are majorly non-aromatic ones. Atoms are bound to one another to create rings in homocyclic and heterocyclic compounds, which are enclosed cyclic structures. Homocyclic compounds are built of rings made of atoms of the same element, whereas heterocyclic compounds are made of rings made of atoms of various elements. This is the primary distinction between homocyclic and heterocyclic compounds. The various elements include sulphur, oxygen, and nitrogen. The molecules pyrrole, furan, and thiophene are examples of heterocyclic compounds. Each only has one heteroatom.
Alicyclic (Cycloalkanes) Compounds
Hydrocarbons called cycloalkanes have one or more carbon rings. These carbon rings are tightly bound to the hydrogen atoms. The minimum number of carbon atoms required to make a cycloalkane is 3. The general formula of cycloalkane is CnH2n. Cycloalkanes include, but are not limited to, cyclobutane (C4H8), cyclooctane (C8H16), cyclohexane (C6H12), cycloheptane (C7H14), and cyclopentane (C5H10).
Aromatic Hydrocarbons
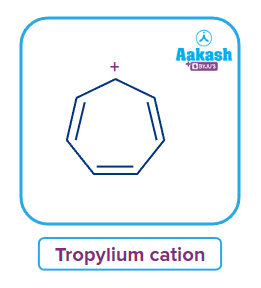
Hydrocarbons that cyclic, planar, and have (4n+2) delocalised -electrons (where ‘n’ is any whole number’) are called aromatic hydrocarbons. They are also known as arenes or aryl hydrocarbons. In the case of benzene, which is an aromatic hydrocarbon, resonance stabilises the benzene ring, and the pi electrons in the ring structure are delocalized. Chemically speaking, the aromatic compounds adhere to Huckel's rule. They should have (4n+2) -electrons in the cyclic structure. They are made up of planar molecules that are conjugated in a ring structure and have delocalised -electron cloud. These substances go by names like aromatics or arenes. So, basically they have alternate single and double bonds.
Examples: Benzene, toluene, etc., They form the sigma bond with planar ring conjugation in aromatic hydrocarbons. Also, tropylium cation is cyclic, conjugated and planar structure with (4n+2) -electrons.
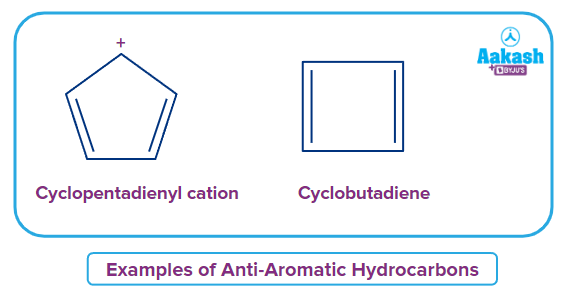
Anti-Aromatic Hydrocarbons
They are cyclic, planar and conjugated rings that contain (4n) -electrons that undergo delocalisation. These are extremely unstable in nature. Hence, they are basically cyclic molecules with a higher energy electron system—due to the existence of 4n -delocalized (or lone pair) electrons.
Examples: Cyclopentadienyl cation, benzene dication, etc., These molecules are cyclic, conjugated and have 4n -delocalized electrons.
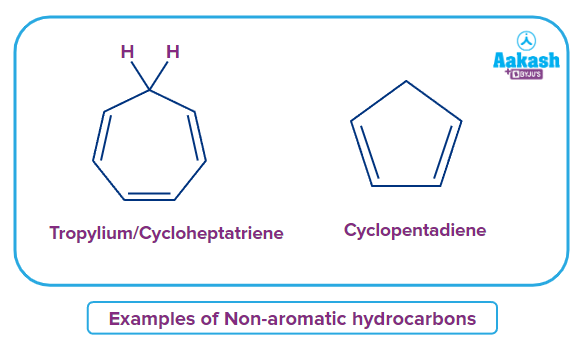
Non-aromatic Hydrocarbons
Non-aromatic hydrocarbons without rings are aliphatic hydrocarbons. Non-aromatic hydrocarbons do not have delocalisation of -electrons and neither are they cyclically conjugated or planar. In fact straight chain alkanes or alkenes can also be termed non-aromatic. Non-aromatic hydrocarbons are essentially non-planar and non-conjugated even if they are cyclic. There is absence of -electron delocalisation. There is no continuous overlapping of p- orbitals in the ring in an aliphatic or non-aromatic cyclic compound.
Example: Cycloheptatriene, cyclopentadiene, etc., They are non-conjugated cyclic rings and does not follow (4n+2) -electron rule (Huckel’s rule).
Heterocyclic Compounds
The ring of a heterocyclic compound contains at least two unique components. On a cyclic ring, the most frequent hetero atoms are oxygen (O), nitrogen (N), and sulphur (S). Heterocyclic compounds include nucleic acid, which is a substance found in the body and is in charge of storing and expressing genetic information. Vitamins are a crucial heterocyclic molecule. Heterocyclic compounds are present in the majority of medicines, pesticides, dyes, and plastics. Majorly there are two types of heterocyclic compounds. Aliphatic heterocyclic compounds and aromatic heterocyclic compounds.
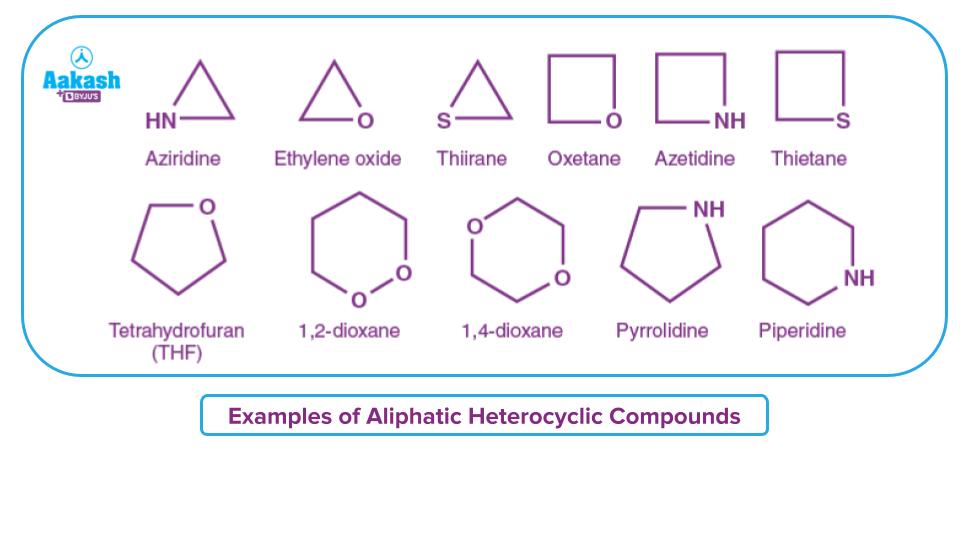
Aliphatic Heterocyclic Compounds
Cyclic heterocycles without double bonds are known as aliphatic heterocyclic compounds. Ring strain mostly influences how aliphatic heterocyclic compounds behave. Examples include tetrahydrofuran (THF), pyrrolidine, dioxane, aziridine, ethylene oxide, etc,.
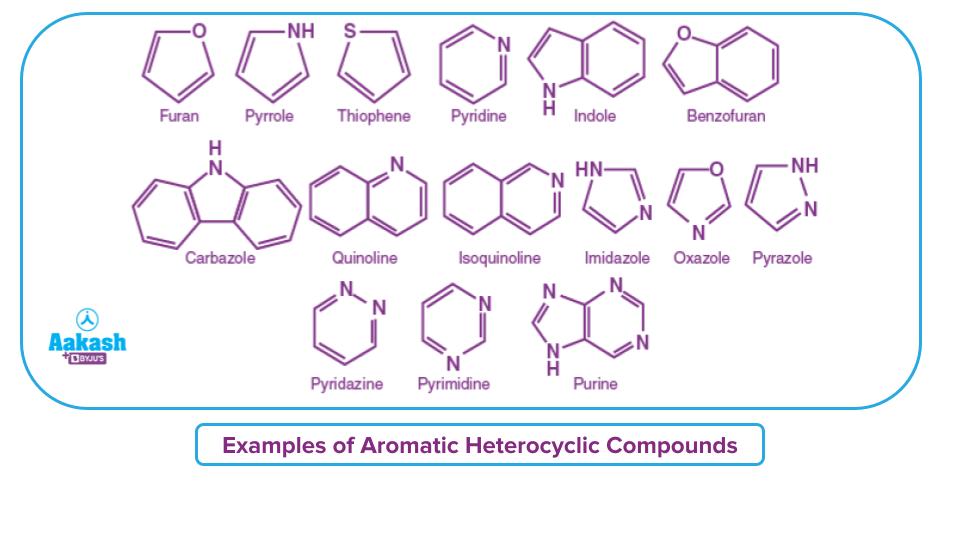
Aromatic Heterocyclic Compounds
Aromatic heterocyclic compounds consist of rings with more than one type of atoms and the rings are cyclic, planar and are conjugated or resonance stabilised with (4n+2) -electrons. To be precise, these heterocyclic rings are aromatic in nature. They have (4n+2) delocalised -electron system and do not contain any sp3 hybridised carbon atoms.
Example: Pyridine, furan, pyrrole, indole, quinoline, etc,.
Comparison between Unsaturated and Saturated Hydrocarbons
|
Saturated Hydrocarbons |
Unsaturated Hydrocarbons |
|
All of the carbon atoms in saturated hydrocarbons are sp3 hybridised. |
All of the carbon atoms in unsaturated hydrocarbons are sp2 or sp hybridised. |
|
Saturated hydrocarbons contain more hydrogen atoms than unsaturated hydrocarbons. |
Unsaturated hydrocarbons contain fewer hydrogen atoms than saturated hydrocarbons. |
|
Hydrocarbons that are saturated have lower chemical reactivity as compared to unsaturated ones. |
The chemical reactivity of unsaturated hydrocarbons is relatively high. |
|
Alkanes and cycloalkanes are typical instances of saturated hydrocarbons. |
Alkynes, aromatic hydrocarbons, and alkenes are typical examples of unsaturated hydrocarbons. |
Uses of Hydrocarbons
- Paints employ hydrocarbons as a solvent.
- These are also employed in the lubrication and grease industries.
- For the manufacture of many medications, hydrocarbons are used.
- Different kinds of polymers are synthesised using hydrocarbons.
- Fuels mostly consist of hydrocarbons.
- Methane is the main component of natural gas.
- Either syngas or ethylene and propylene are created from ethane and propane.
Recommended Video
Hydrocarbons Class 11 Chemistry (Ch-13) | JEE Main 2022 Important Topics | JEE Exam Preparations
JEE Main 2022: Hydrocarbons Class 11 Chemistry (Top 12 Most Important and Expected Questions)
Hydrocarbons Class 11 Chemistry (Ch-13) | JEE Main Previous Year Questions | JEE 2022 Online Classes
JEE Advanced 2022: Hydrocarbons JEE Advanced Problems/Questions | JEE Advanced Chemistry Solutions
Practice Problems
1. Mention one chemical test to distinguish internal and terminal alkynes.
Solution: Acidic hydrogen is present in terminal alkynes. Tollen's reagent, which is essentially an ammoniacal silver nitrate solution, produces a white precipitate (silver alkynide) with terminal alkynes while having no effect on non-terminal alkynes.
Tollen’s Reagent Silver Alkynide (white ppt.)
This test can be used to distinguish between internal and terminal alkynes.
2. Select the correct order of acidities.
a.
b.
c.
d.
Answer: A
Solution: Triply-bonded carbons of ethyne and propyne are sp hybridised. The terminal hydrogen atoms in ethyne and propyne are highly acidic due to the high % s-character in the sp hybridised orbitals, making the carbon atoms highly electronegative.
Also, the conjugate bases obtained from ethyne and propyne are highly stable as the negative charge is present on the sp hybridised carbon. The conjugate base of ethene is not that stable as the negative charge is present on the sp2 hybridised carbon. Hence, ethene is less acidic than ethyne and propyne.
In propyne, one sp hybridised carbon is attached to −CH3 which destabilised the negative charge of its conjugate base, decreasing its acidity in comparison to ethyne.
The conjugate base of ethane is highly unstable as the negative charge is destabilised by the +I effect of −CH3, making ethane least acidic.
Hence, the correct order of acidity is
H-C≡C-H > CH3−C≡C−H > H2C=CH2 > CH3−CH3
So, option A is the correct answer.
3. Mention properties of aromatic hydrocarbons:
Solution: The following are the properties of aromatic hydrocarbons.
- Aromatic hydrocarbons exist in the gaseous as well as liquid forms.
- Natural aromatic hydrocarbons have no colour.
- Water does not dissolve aromatic hydrocarbons.
- Electrophilic substitution reactions are undergone by aromatic hydrocarbons.
- These hydrocarbons can occasionally exhibit addition and oxidation processes as well.
- Aromatic hydrocarbons release a yellow, sooty blaze when burned.
- Which among the following is a cycloalkane?
- Benzene
- Propyne
- Pentane
- Cyclopentane
Answer: D
Solution: The suffixes ene, yne and ane are used for IUPAC names of alkene, alkyne and alkane, respectively. Cycloalkanes have the prefix ‘cyclo’ in front of their IUPAC names and ‘ane’ as the suffix. The only option that contains the suffix -ane and the prefix ‘cyclo’ is option D.
So, option D is the correct answer.
Frequently Asked Questions – FAQ
1. Are alkynes polar or nonpolar in nature?
Answer: Alkynes are unsaturated, non-polar hydrocarbons having properties similar to those of alkanes and alkenes. Alkynes are rarely soluble in polar solvents, soluble in organic solvents, and insoluble in water.
2. How is the hydration of alkynes carried out if they are not soluble in water?
Answer: Generally, alkynes and water do not mix under normal conditions. As a result, alkynes only react with water when acid is used as the catalyst. In order to produce enols that spontaneously tautomerize to ketones in the presence of diluted sulfuric acid and mercuric sulphate catalyst, alkynes are induced to undergo acid-catalysed hydration. For example: oxymercuration is a process where in the presence of catalyst water electrophilic addition of water occurs.
3. What is the composition of crude oil?
Answer: Crude oil is a very complex mixture of aromatic, paraffinic, and cyclo paraffinic (naphthenic) hydrocarbons with traces of nitrogen and oxygen molecules and a low sulphur content.
4. What type of hydrocarbon does petrol consist of?
Answer: Hydrocarbons, or compounds made exclusively of carbon and hydrogen, make up the majority of petroleum. Alkanes (paraffins), cycloalkanes (naphthenes), and aromatic hydrocarbons are the most prevalent constituents. Although small numbers of shorter or longer molecules may be present in the mixture, they typically have between 5 and 40 carbon atoms per molecule.


