-
Call Now
1800-102-2727
Chemical Properties of Alkenes- Addition of Hydrogen Halides (HX), Acid-Catalyzed Hydration, Hydroboration-Oxidation Reaction and Oxymercuration-Demercuration Reaction, Reaction with silver oxide, and Polymerization, Practice Problems and FAQs
Many types of hair growth, cosmetics, hygiene goods, such as hair styling products, fragrances, deodorants, contain alcohol. Alcohol is utilized because of its high volatility (it vanishes almost instantly after application), drying, refreshing, and antibacterial characteristics. It improves the deposit of foundation pigments on the skin. But what is the source?
The answer is Alkene. Alkenes can be converted to alcohol by three different chemical processes. Alkene is also reacts with many other reagents to give interesting products.some of which are discussed here.

Table of Contents
- Addition of Hydrogen Halides (HX)
- Addition of water
- Hyboration-Oxidation Reaction
- Hydration by Oxymercuration-Demercuration:
- Reaction of an alkene with dry silver oxide
- Polymerization
- Practice Problems
- Frequently Asked Questions
Addition of Hydrogen Halides (HX)
Hydrogen halides (HCl, HBr, HI) when added to alkenes give alkyl halides. This is known as the hydrohalogenation process. It is an electrophilic addition reaction.
Alkene + HX Alkyl halide
Example:
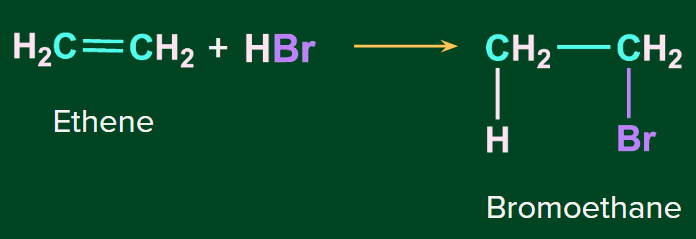
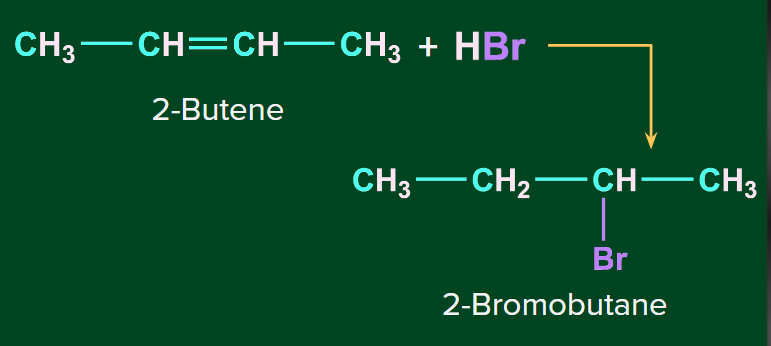
Addition of HBr to symmetrical alkenes
The addition reaction of HBr to symmetrical alkenes takes place by an electrophilic addition mechanism in which the hydrogen ion gets attached to one carbon and bromide gets attached to the second carbon of the double bond.
Addition of HBr to unsymmetrical alkenes
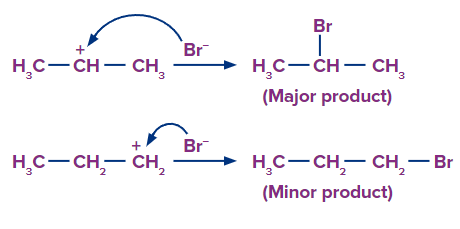
When HBr is added to the unsymmetrical alkenes, there are two possibilities of the addition of HBr to propene (unsymmetrical alkene) in which double bonds can break in such a way that one carbon gets the negative charge and the other gets the positive charge. The breaking of the bond takes place in such a way that the bond-forming carbocation should be the most stable.
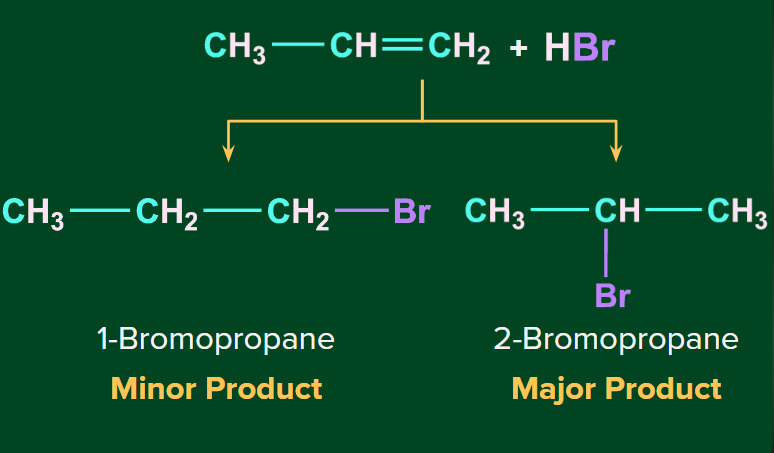
Under normal conditions the major product formed upon the addition of HBr to an unsymmetrical alkene, follows Markovnikov’s rule (The rule states that the negative part of the addendum (adding molecule) gets attached to that carbon atom which possesses a lesser number of hydrogen atoms).
Mechanism (Markovnikov Addition)
Step 1: The H+ ion attacks the double bond (electrophilic addition of H+) and gets attached to the carbon having more hydrogen atoms.
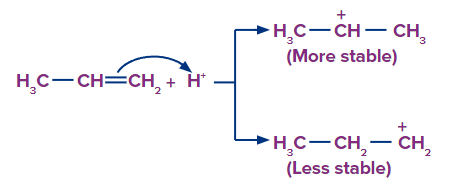
Step 2: Since, the 2o carbocation is more stable than 1o carbocation, so the stable carbocation will be available more for the attack of and the product formed from 2o carbocation is the major product.
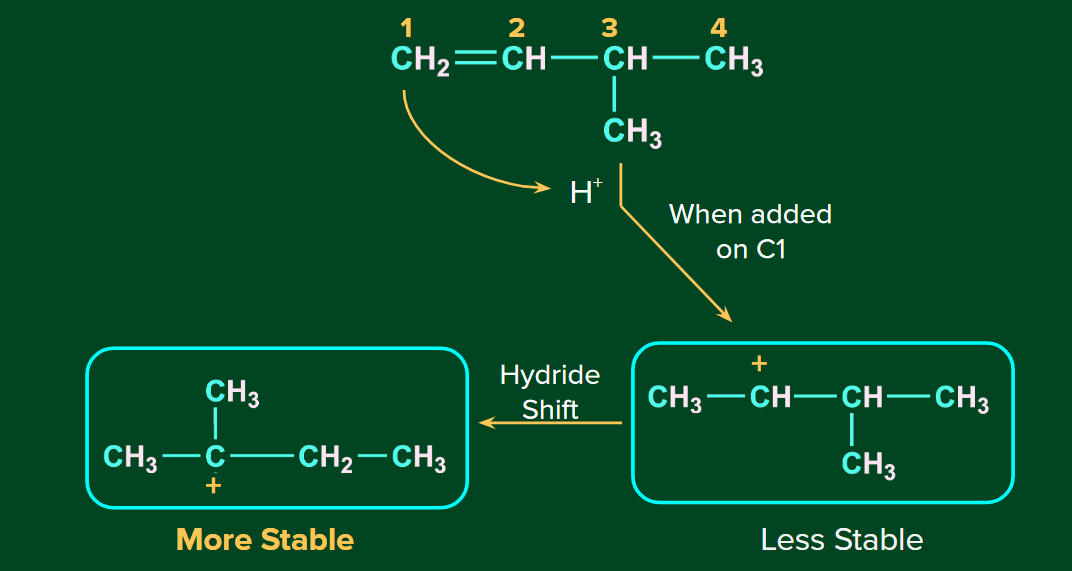
Rearrangement of Carbocation
The H+ ion attacks the double bond and it gets attached to the carbon having more hydrogen atoms. The 2o carbocation undergoes rearrangement (1,2-hydride shift) to form a more stable 3o carbocation. Finally, ion attacks on the 3o carbocation to form the major product.
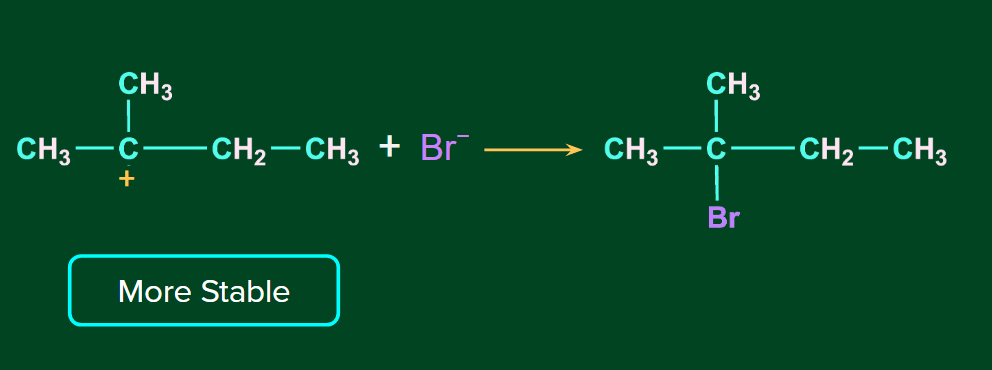
Order of reactivities of hydrogen halides
As we move down the group, the bond length of HX increases and the bond strength decreases (i.e., the H-X bond becomes weak and can be easily dissociated). Thus, the reactivity of hydrogen halides towards addition reactions increases down the group.
HF < HCl < HBr < HI
Mechanism (Anti-Markovnikov Addition)
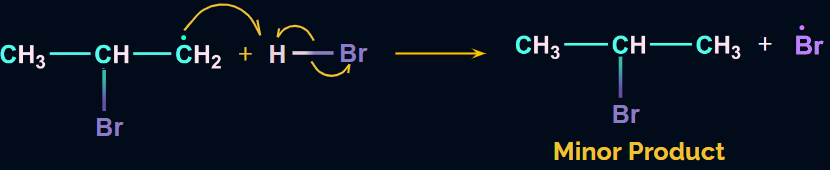
When alkenes are treated with HBr in the presence of peroxides, anti-markovnikov’s addition occurs such that the H-atom of HBr gets attached to the C-atom with the fewer H-atoms. It is also known as the peroxide or the Kharasch effect.
The addition of HBr to an alkene in the presence of peroxide takes place through a free radical mechanism.
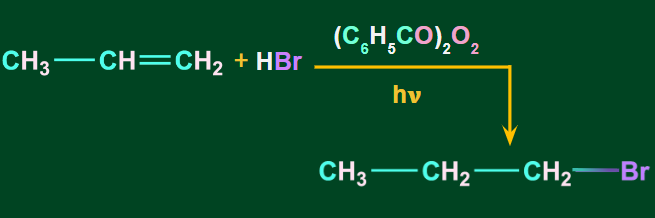
Where (C6H5CO)2O2 is benzoyl peroxide
The mechanism is given as follows:
- Chain initiation
Step 1: The peroxide bond of RCO–O–O–CO–R breaks into radicals (i.e., RCOO) in the presence of sunlight, which is then followed by the removal of carbon dioxide and the formation of alkyl radical (i.e., R).
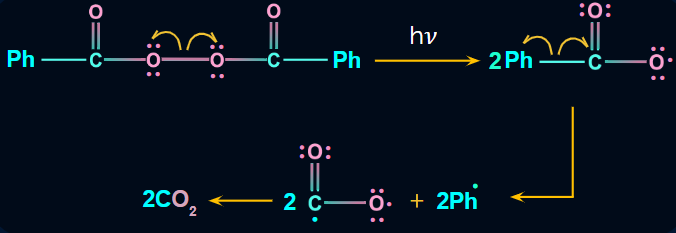
Step 2: Now, the alkyl free radical (R) (i.e., phenyl radical) attacks on HBr and results in the formation of R−H (i.e., benzene in benzoyl peroxide) and Br radicals.
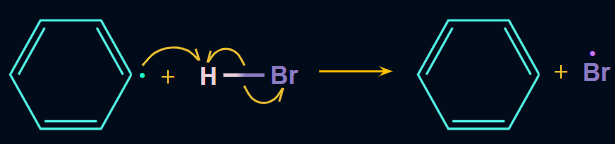
- Chain propagation
The order of stability of the free radicals is . The two intermediates formed are given and we know that a 2o free radical is more stable than a 1o free radical.
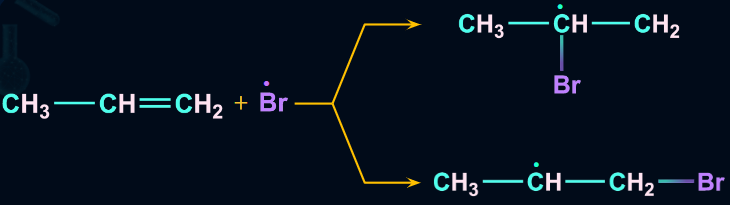
- Chain Termination
The hydrogen radical formed from HBr will attack the 2o free radical to give the major product. Hence, in the major product, Br will be attached to the carbon with more hydrogen atoms, which is in contradiction to Markovnikov’s rule. Hence, it is an anti-Markovnikov addition.
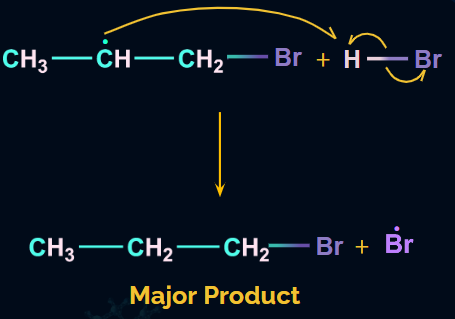
Addition of Water
There are three methods by which water can be added to an alkene
- Acid-catalyzed Hydration
- Hyboration-Oxidation Reaction
- Oxymercuration-Demercuration Reaction
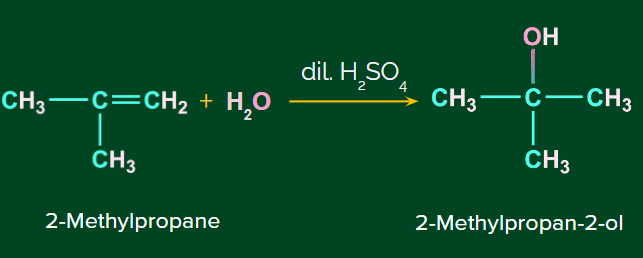
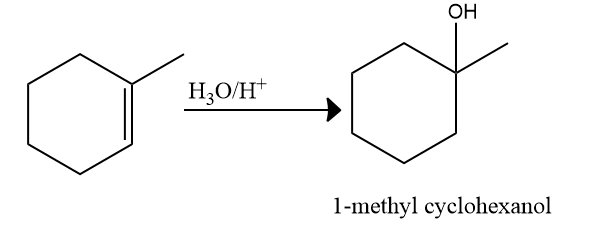
Acid catalyzed hydration:
This is also known as the hydration of alkenes. Addition of (acid catalyzed hydration) to alkenes is a Markovnikov addition reaction.
Example: Addition of H2O to isobutene
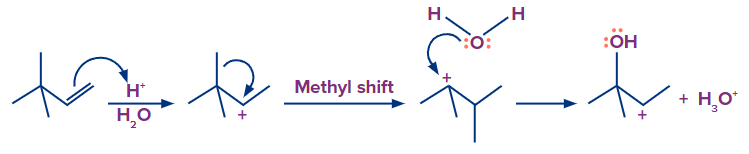
Mechanism of Acid-catalyzed hydration:
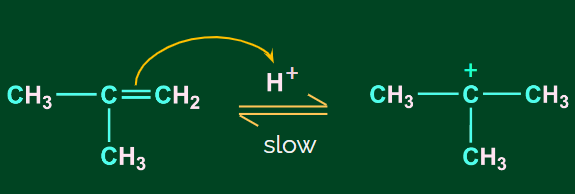
Step 1: Protonation and formation of carbocation
When the double bond breaks, it forms a stable carbocation. Here, a tertiary carbocation is formed
instead of a primary carbocation. This is because the tertiary carbocation is more stable than the
primary carbocation.
Step 2: Nucleophilic attack of H2O
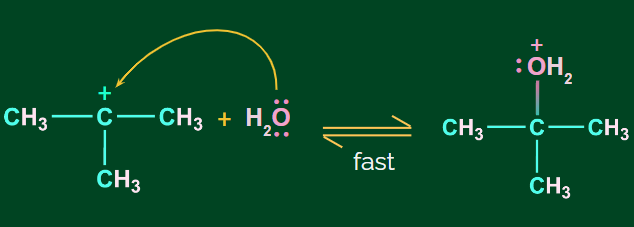
Step 3: Deprotonation to form alcohol
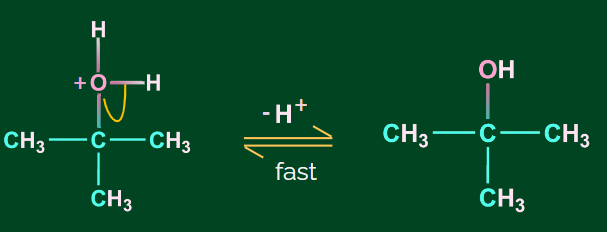
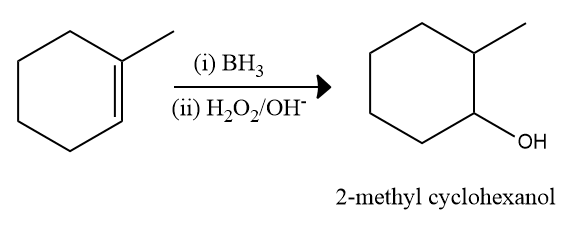
Hyboration-Oxidation Reaction: The hydroboration oxidation reaction is an organic chemical reaction that is used to convert alkenes into primary alcohols or alkynes into ketones or aldehyde.This is accomplished through a net addition of water (across the entire double bond)using an anti-Markovnikov Rule.
Let us consider the example of Hyboration oxidation of alkene hex-1-ene.
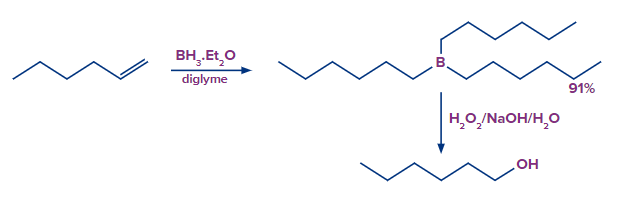
Mechanism of Alkene Hydroboration-Oxidation
The mechanism of hydroboration oxidation can be thought of as an anti-Markovnikov reaction in which a hydroxyl group attaches itself to the less substituted carbon.
Characteristics of Hydroboration
- Stereospecific -syn addition
- Anti Markovnikov mechanism
- Primary alcohol formation
The conversion of alkenes into neutral alcohols takes place here. The entire reaction can be broken down into two steps, as explained below.
- The Hydroboration Process
The first step is to add borane in the form of BH3 to the given double bond. This results in the transfer of a hydrogen atom to the carbon atom next to the carbon atom bonded with the boron atom. The hydroboration step is now repeated twice, yielding three alkenes attached to the boron atom from the starting BH3.
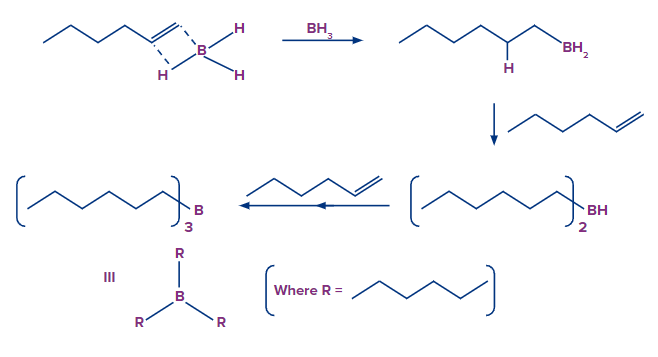
- The Oxidation Process
Now that the trialkyl borane has been produced, the second step in the hydroboration process can begin. The boron atom is attacked in this step by the hydroperoxide ion, which is nucleophilic in nature. The R group, along with its electron bond pair to the adjacent oxygen atom, is now rearranged.
The ion of hydroxide has now been removed. This process is repeated three times to produce trialkyl borate as the product. This trialkyl borate is now treated with water to produce the required primary alcohol. This step of the mechanism is illustrated below.
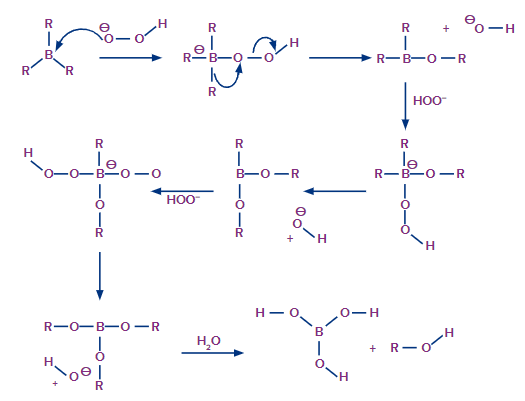
Hydration by Oxymercuration-Demercuration:
Alkenes can be converted to alcohol using the oxymercuration-demarcation reaction. An alkene is treated with mercuric acetate in a Tetrahydrofuran–water solution to produce a product, which can then be reduced with NaBH4 to produce alcohol. Markovnikov's rule operates in this reaction.
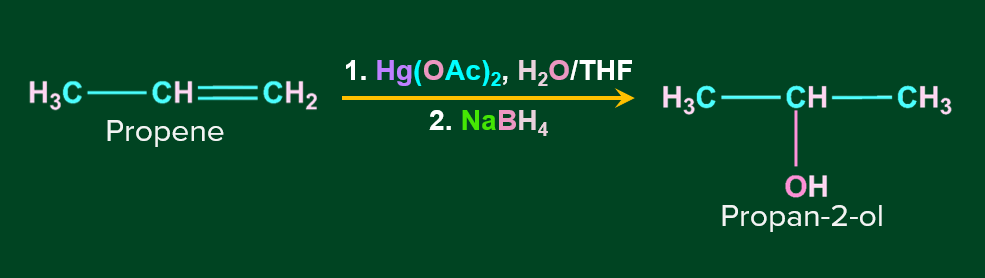
Mechanism of Oxymercuration-Demercuration
The mechanism of this reaction follows Markonikov's regioselectivity rule, with the hydroxyl group joining the most substituted carbon atom and the hydrogen atom joining the least substituted carbon atom.
Characteristics of Oxymercuration-Demercuration
- Stereospecific -anti addition
- Markovnikov mechanism
The reaction mechanism is depicted below.
Step 1: In this step, the nucleophile C=C bond attacks the electrophile Hg, resulting in the acetate ion leaving as the leaving group and the formation of cyclic mercurinium ion.
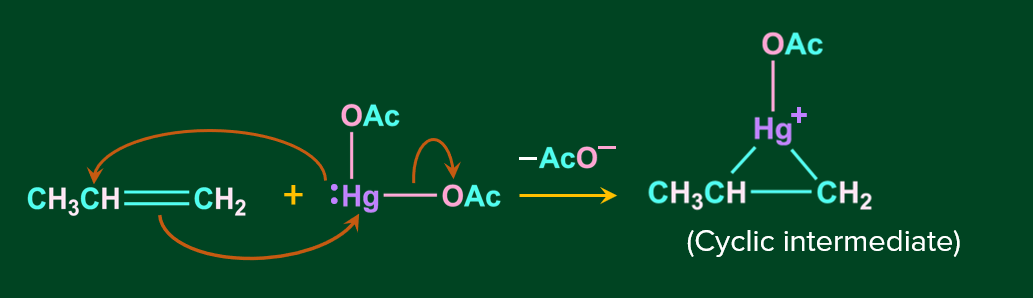
Step 2: In this step, the nucleophile attacks one of the carbons linked to Hg, causing the C-Hg bond to cleave. It is stereospecifically -anti addition.
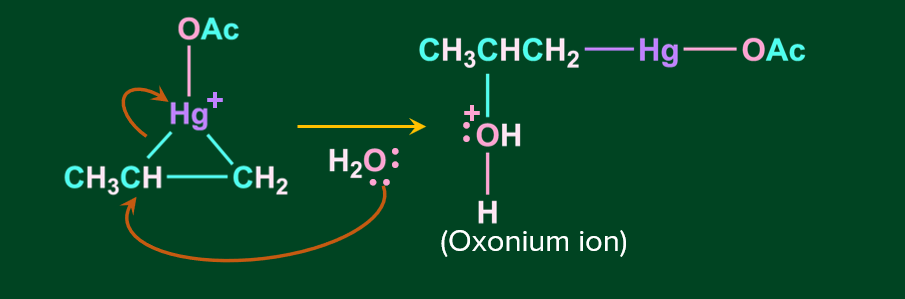
Step 3: In this step, oxonium ion is deprotonated in the presence of a base acetate ion, resulting in alcohol.
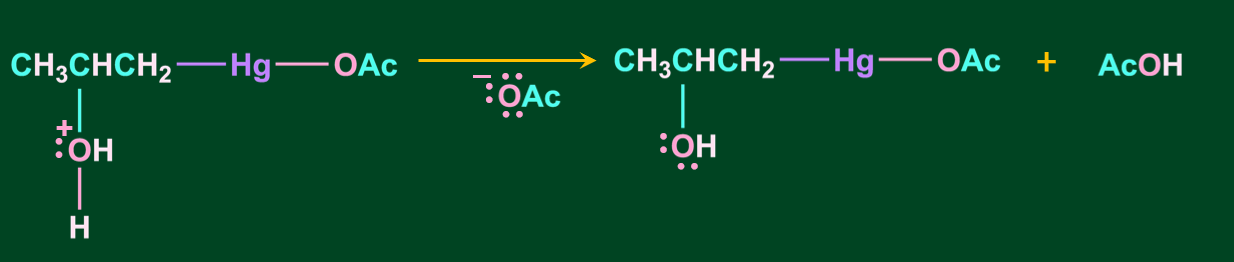
Step 4: In the final step, the addition of sodium borohydride replaces the acetyl mercury with a hydrogen atom, resulting in the formation of alcohol via a new C-H bond.
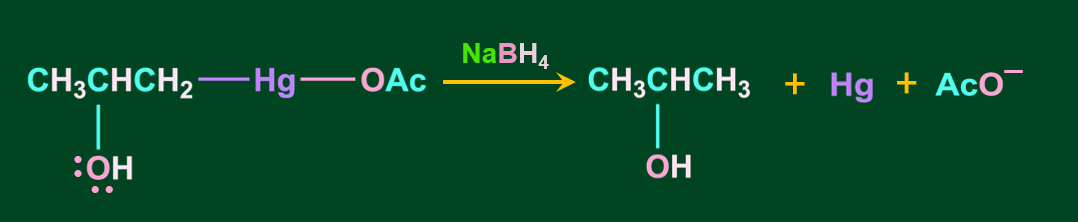
|
Reactions |
Stereochemistry |
Product |
Rule |
|
Acid catalyzed Hydration |
Stereospecific -anti addition |
Secondary, tertiary |
Markovnikov mechanism with rearrangement |
|
Hyboration-Oxidation Reaction |
Stereospecific -syn addition |
Primary |
Anti-Markovnikov mechanism |
|
Oxymercuration-Demercuration Reaction |
Stereospecific -syn addition |
Secondary, tertiary |
Markovnikov mechanism |
Reaction of an alkene with dry silver oxide
The majority of epoxides are formed by reacting alkenes with peroxide-containing reagents, which donate a single oxygen atom. Metal complexes are also effective epoxidation catalysts. When alkene reacts with dry Ag2O which is prepared in situ from silver and oxygen, they undergo Epoxidation.
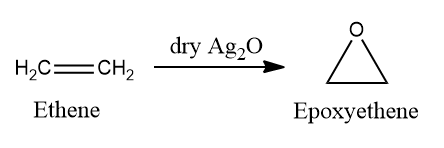
High temp is used to form nascent oxygen ([O]) which follows a free radical mechanism to form an epoxide.
Epoxyethane is a heterocyclic compound. These compounds are cyclic structures in which one (or more) of the ring atoms is a hetero atom, that is, an atom of a non-carbon element.
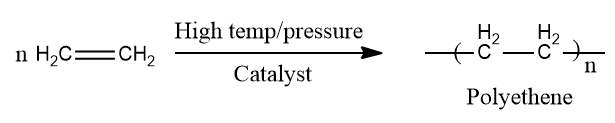
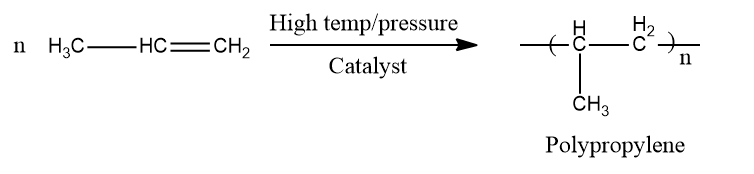
Polymerization
You've probably seen polythene bags and sheets. Polythene is made by combining a large number of ethene molecules under high pressure and temperature in the presence of a catalyst. Polymers are the big molecules that result from this process. The polymerisation is the name for this reaction.
Practice Problems
Q1. What will be the major product when 5-methyl hex-2-ene is treated with acid-catalysed water?
A. 5-methyl hexan-2-ol
B. 5-methyl hexan-3-ol
C. 5-methyl hexanal
D. 5-methyl hexan-2-one
Solution: 5-methyl hex-2-ene on reaction with H2O in the presence of dilute H2SO4 yields 5-methyl hexan-2-ol and 5-methyl hexan-3-ol as the products. The carbocations formed from the addition of hydrogen ions are
secondary carbocations. The latter secondary carbocation is more stable because it has 5 hyperconjugation structures with respect to 5 hydrogens whereas the former carbocation is stabilized by 4 hyper conjugating structures with respect to 4 hydrogens. Hence, the major product is hexan-2-ol.
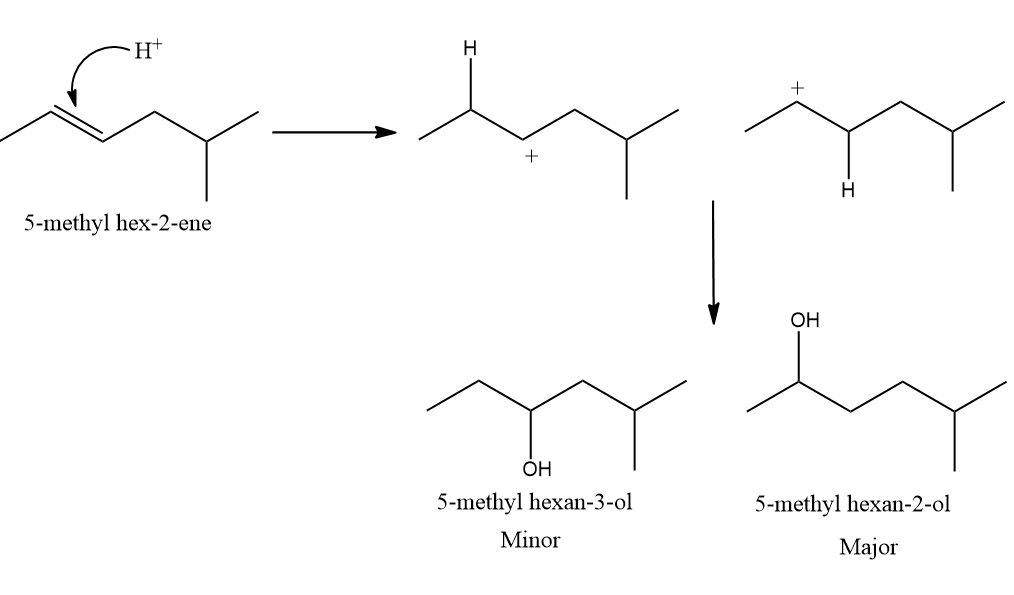
Hence, the correct answer is an option (A).
Q2. In which case will hydroboration oxidation and acid hydration produce different products?
(A) ![]()
(B) ![]()
(C) CH2=CH2
(D) CH3-CH=CH-CH3
Solution: The hydroboration oxidation reaction converts alkenes into alcohol by an organic chemical reaction. A two-stage technique, which involves a hydroboration step and an oxidation step, is used to produce this. Using an anti-Markovnikov Rule, a net addition of water is achieved.
Acid-catalyzed hydration is a chemical reaction in which water is added to an unsaturated substrate while an acid catalyst is present. The hydration of ethene is one example.
Cyclohexene (option B), CH2=CH2 (option C) and CH3-CH=CH-CH3 (option D) all are symmetrical alkene. Hence, the Markovnikov Rule is not applicable in this case. As products formed from Markovnikov or anti-Markovnikov are the same compound.
In option (A), the unsymmetrical alkene will yield a different product concerning the reagent and can be shown as
Hence, the correct answer is option (A).
Q3. Hydrocarbon A adds hydrogen to produce methyl cyclopentane in the presence of a platinum catalyst. Only a single Bromo compound is generated when hydrogen bromide is introduced to A instead of hydrogen?? Predict A.
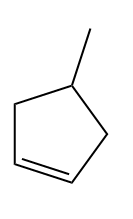
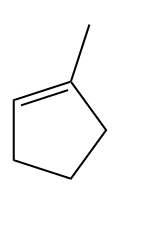
- Both (a) and (b)
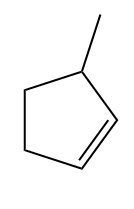
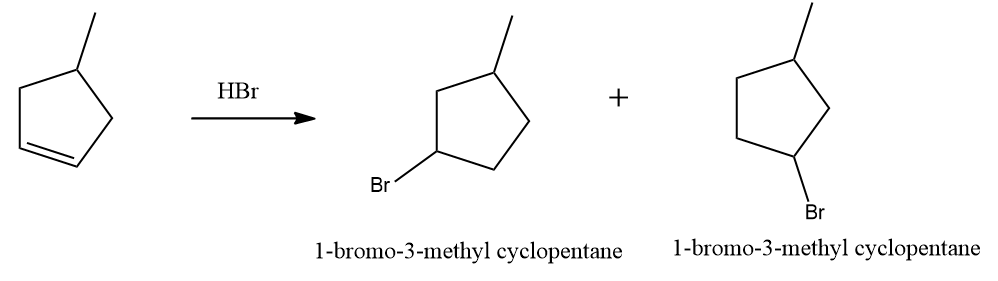
Solution: As in option (A), given alkene is a symmetrical alkene, only one product will be obtained on the addition of HBr,
As in option(B), alkene is an unsymmetrical alkene, more than one product is formed. 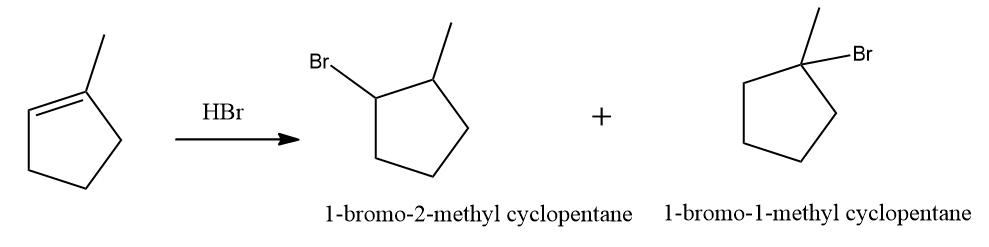
As in option(D), alkene is an unsymmetrical alkene, 2-Bromohexane will be formed as the major product and 1-Bromohexane will be formed as a minor product.
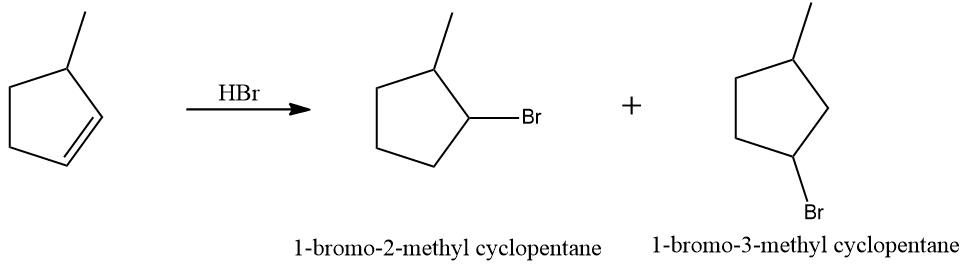
Since only one bromo compound is formed as the product in the case of 4-methyl cyclopent-1-ene. Hence option (A) is the correct answer.
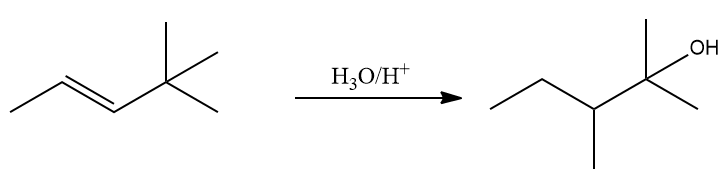
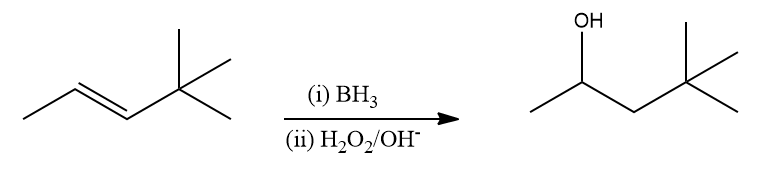
Q4. Which of the following reactions is/are correct?
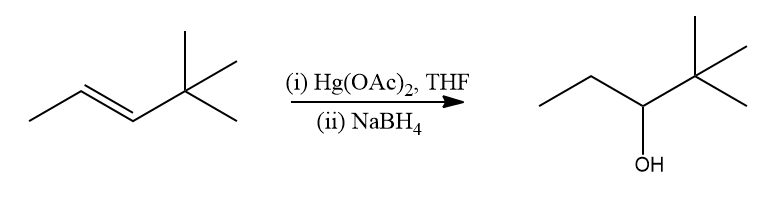
- All of these
Solution: Alcohol can be produced by the oxymercuration-demarcation process from alkenes. An alkene is treated with mercuric acetate in a THF-water solution to create a product, which can subsequently be reduced with NaBH4 to create alcohol. Markownikov's rule states that addition takes place in this reaction. Hence, in option (C), the given reaction is correct.
Alkenes can be changed into neutral alcohols via the hydroboration oxidation reaction, and alkynes can be changed into ketones or aldehydes. This is done via a two-stage process that consists of a hydroboration step and an anti-Markovnikov Rule-based oxidation step. Hence, in option (B), the given reaction is correct.
Alkenes undergo an addition reaction known as a Markovnikov reaction when (acid catalysed hydration) is introduced. In option(A), the given reaction is true
So, as per the explanation, all the given reactions are correct. So, the correct answer is option (D).
Frequently Asked Questions
Q1. What applications does alkene have?
Answer: They are used in the production of plastics such as polythene for buckets, bowls, and bags and polystyrene for car battery cases and refrigerator parts. They are used in the production of ethane-1,2-diol, which is used as an anti-freeze agent in automobile radiators.
Q2. What are the applications of the Hydroboration and Oxymercuration-Demercuration reactions?
Answer: It is used in the production of alcohol from alkenes. It produces more stereospecific and regioselective alcohols than other oxidation reactions used in alcohol formation.
Q3. Why are Lindlar catalysts' lead salts and quinoline 'Poisoned'?
Answer: Typical palladium catalysts have high catalytic activities that can reduce even double bonds. Alkanes can be formed during the hydrogenation of alkynes using such catalysts (the alkene products undergo further hydrogenation under the influence of the catalyst).The poisoned Lindlar catalyst is incapable of reducing double bonds. As a result, using this catalyst in the hydrogenation of alkynes does not result in the formation of alkanes.
Q4. What exactly is the distinction between dehydration and dehydrogenation?
Answer: These terms have perplexed us all. The main difference between hydration and hydrogenation is that hydration involves adding water molecules to an organic complex whereas hydrogenation requires adding a hydrogen molecule.


