-
Call Now
1800-102-2727
Chemical Equilibrium- Equilibrium, Types, Practice Problems and FAQs
Is it possible to attain equilibrium, with the two forces in motion? Well definitely yes, it can be.
Let's take one bucket with a tap at the bottom and filled by the same tap. The rate of inflow of water equals the rate of outflow of water in a bucket. The volume of water in the bucket remains the same. This is an example of dynamic equilibrium.
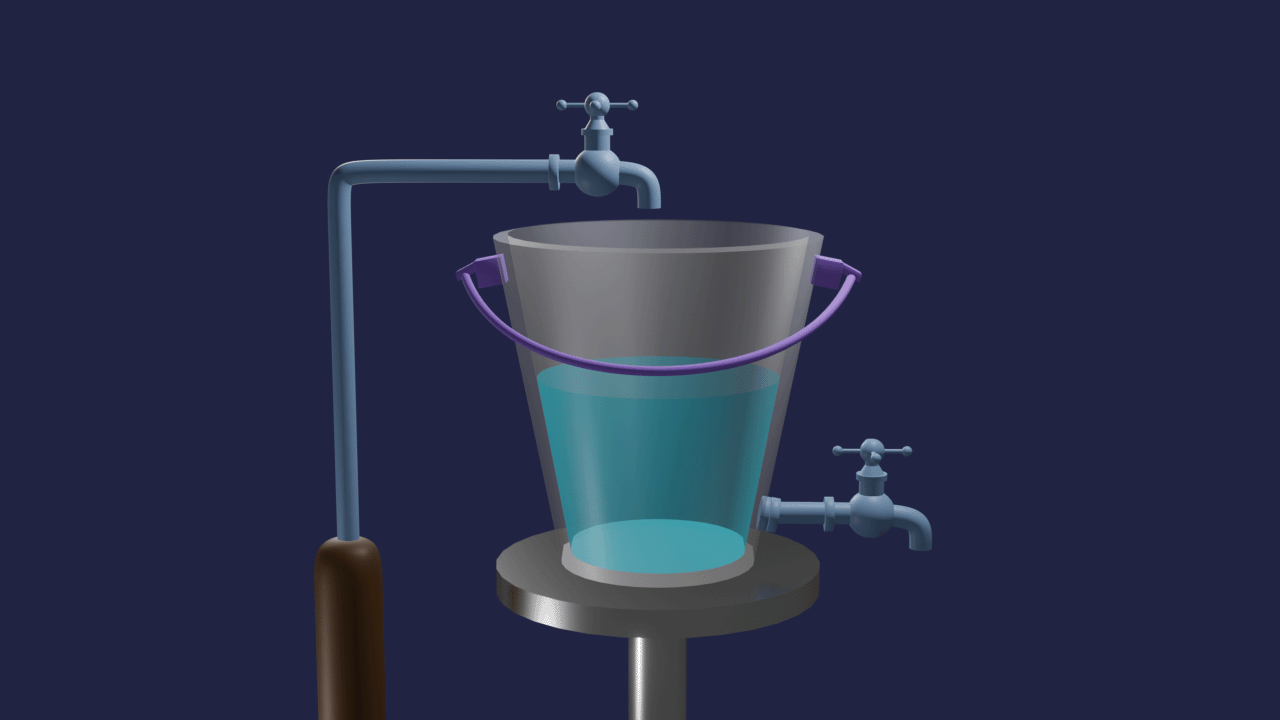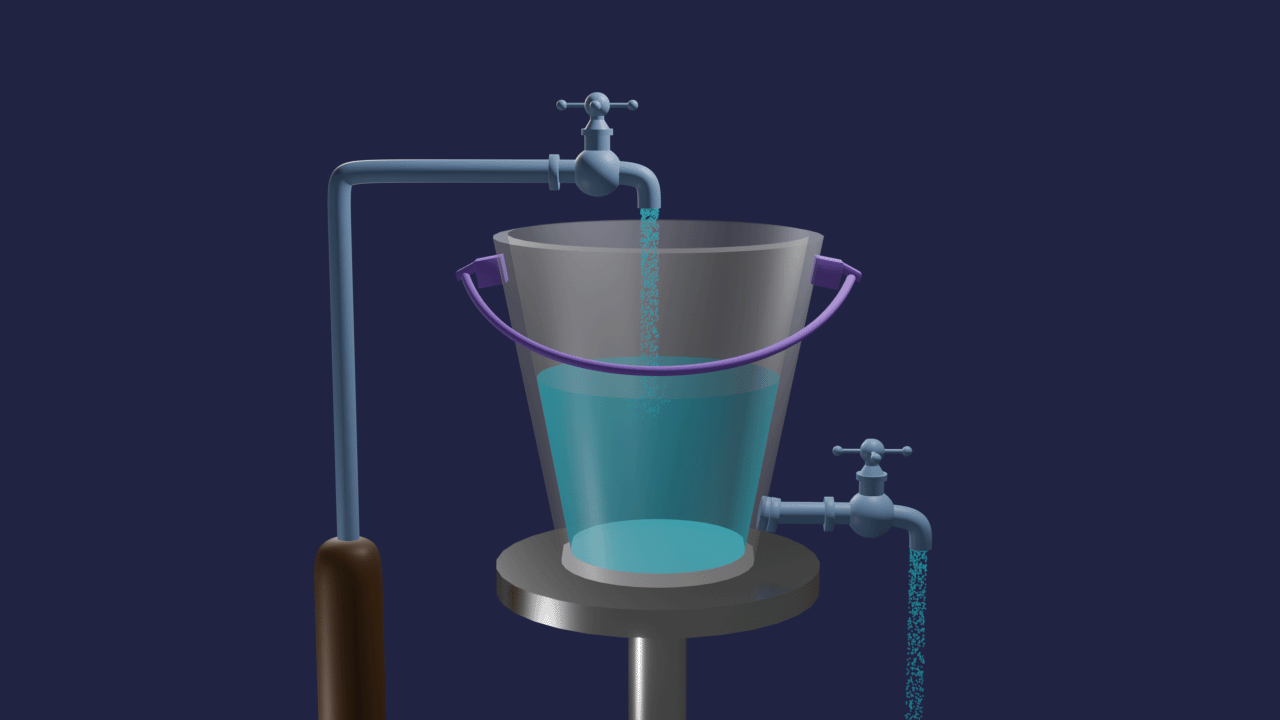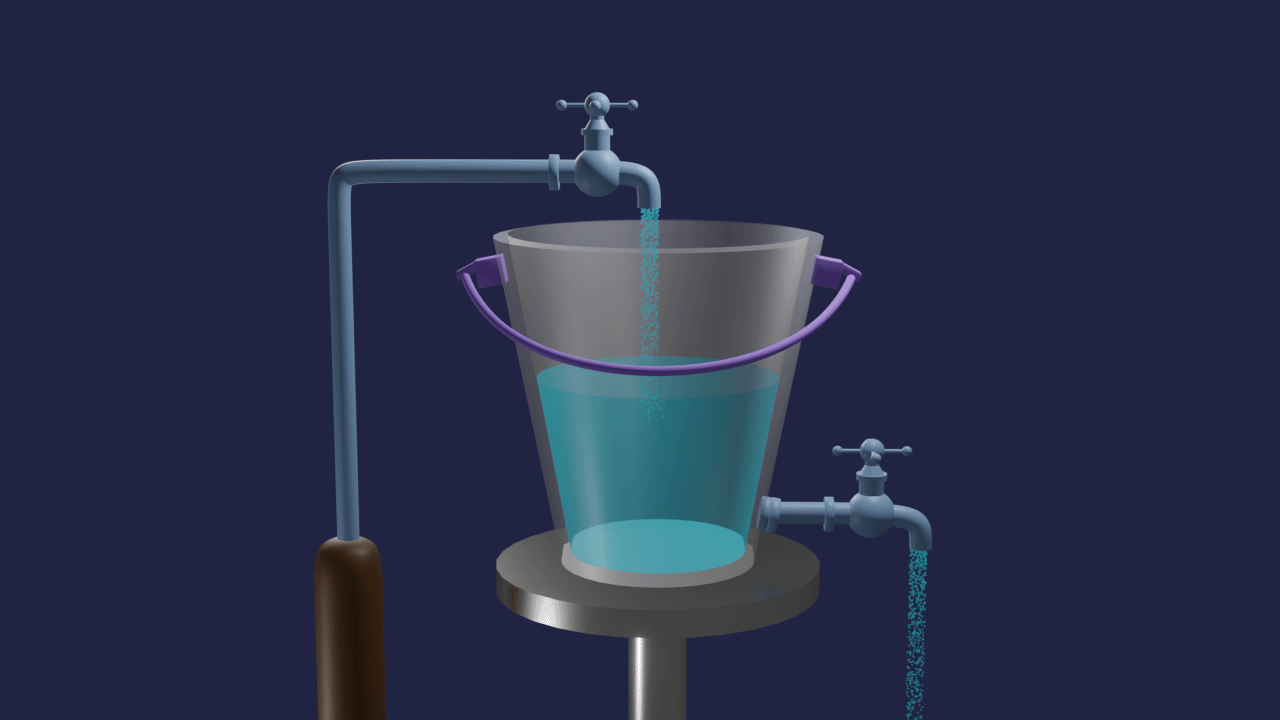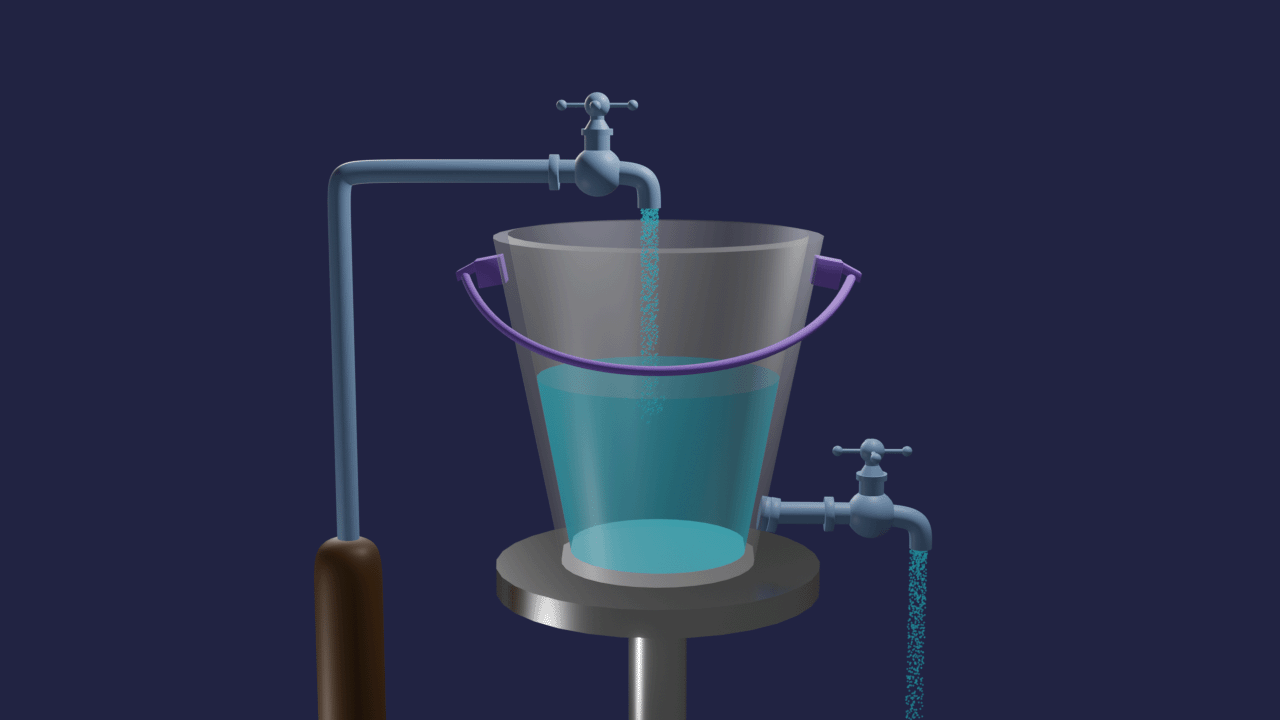

Similar to this, many chemistry processes struggle to complete but produce mixtures of reactants and products in a fixed proportion. Such reactions have significant effects on the industry's output of chemicals, product purity, and cost-effectiveness of production, among other things.
Although it seems that these reactions are slowing down, they actually continue. If you could see what was happening at the microscopic resolution, you would observe that the reactants and products are forming each other at the same rate.
Table of Content
- Chemical equilibrium
- Characteristics of Chemical Equilibrium
- Homogeneous equilibrium
- Heterogeneous Equilibrium
- Practice Problems
- Frequently Asked Questions-FAQs
Chemical equilibrium
In a state of chemical equilibrium, reactants convert into products (by a forward reaction), and products also react to produce other products (by a backward reaction). So two active, opposing, reversible reactions are involved in a dynamic equilibrium known as a chemical equilibrium.
This can be comprehended with the aid of a generic reaction. Consider the reversible reaction between reactant R and product P in the following example:
R⇌P
Initially, there is only the reactant. The backward response rate (rb) thus equals zero. On the other hand, the product is produced over time, and its concentration increases along with it. The rate of backward reaction increases as a result. The rate of forward reaction (rf) consequently began to decrease. The forward and backward rates are equal after a certain amount of time (t).
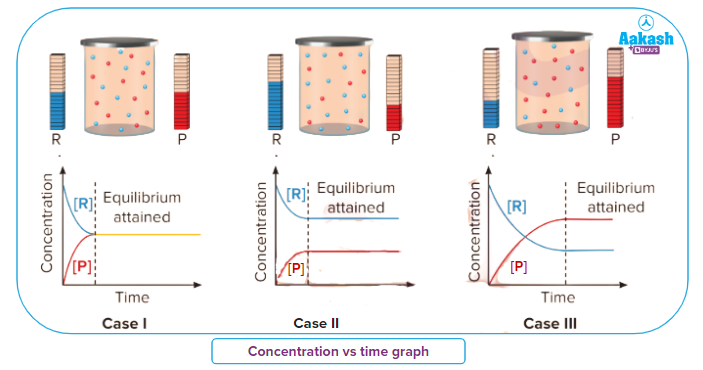
The amount of reactants and products after the attainment of equilibrium,do not change and remains at a constant value. Three types of relative concentration between reactants and products can be expected..
Situation I: [R] = [P]
Situation II: [R] > [P]
Situation III: [R] < [P]
Characteristics of Chemical Equilibrium
- In a closed system, the equilibrium is dynamic. Both the reactions (forward or backwards) continue to proceed..
- At equilibrium, the forward reaction rate and the backward response rate are equal.
- The presence of a catalyst has no impact on the equilibrium state, but depending on the type of catalyst, it changes both rate of reaction in the same way.
- Their concentrations are constant while the reactants and products are in equilibrium.
- The free energy change (G) is equal to zero in an equilibrium state.
- Equilibrium is achieved in its own time by the system.
Chemical Equilibrium can be classified into one more category as
- Homogeneous equilibrium
- Heterogeneous equilibrium
Homogeneous equilibrium
All reactants and products are in the same phase in homogeneous equilibrium.
Example
PCl5(g) (yellow colour) is the sole substance present in the container at first. PCl5(g) decomposes when heated. PCl3(g)(blue colour) and Cl2(g) (orange colour) begins to appear in the beaker. The homogeneous equilibrium is reached after time t.
All reactant and products are in gaseous phase(Phases are identified by their boundaries) and cannot be distinguished. So, they are in homogenous equilibrium.
.

Other examples
Note:
(i) Because gasses are miscible with one another, a gaseous mixture is always in a state of homogenous equilibrium.
(ii) Depending on the miscibility of the liquids present, liquid can either exist in homogeneous equilibrium or in heterogeneous equilibrium.
(iii) Because solids are immiscible with one another, they are always in a state of heterogeneous equilibrium.
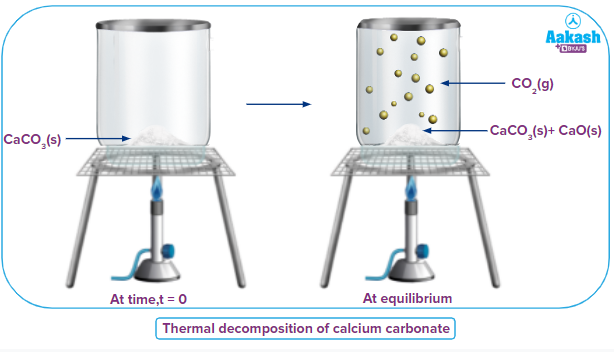
Heterogeneous Equilibrium
The reactants and products are in more than one phase in heterogeneous equilibrium.
Example:
In the reaction, , the reactants and products are in different
phases (solid and gas).
Related Video Applying Concepts: Introduction to Chemical Equilibrium | Chemistry | JEE
Practice Problems:
Q1. Which of the following graphs most accurately depicts the equilibrium A⇌B?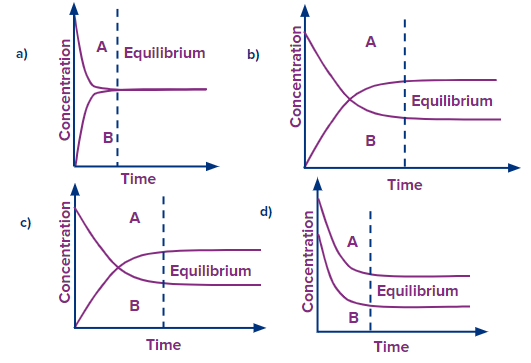
Solution: In graph (A), (B) and (C), a drop in the reactant concentration and an increase in the product concentration are the same.
Graph (D) cannot be the proper graph because the concentrations of both the reactant and the product are falling.
Hence, Right answers are (A), (B) and (C).
Q2. The production of ND3 is carried out under the same partial pressure and temperature circumstances as for NH3. The equilibrium mixtures of H2, N2, NH3, and D2, N2, and ND3 are mixed and set aside for a while. Will equilibrium mixtures react and if yes then how?
Solution: Yes, The equilibrium mixtures will react and form a number of new species.
When the two mixtures are combined, along with these reactions, H2 and D2 combine to produce HD via the reaction:
The produced HD also reacts with N2 to form NHD2 and NH2D, as seen below:
Q3. How many of the reactions below are homogenous and reversible?
A.
B.
C.
D.
Solution: If all the reactants are in the same phase, then reaction is known as in homogeneous equilibrium. In (A) and (B), all the reactants and products are in the same phase. Hence these reactions are in homogeneous equilibrium and reversible in nature.
Q4. Which of the following assertions regarding the reaction is true?
(A) Achieving equilibrium is feasible.
(B) The quantities of reactants and products may vary at equilibrium.
(C) A homogeneous equilibrium
(D) Equilibrium in an environment with heterogeneity
Because equilibrium can be reached in reversible reactions, this is the solution. Therefore, the (A) is correct.
This is also true for (B), as the concentrations of the reactants and products may vary at equilibrium.
Since all of the reactants and products are gasses, it is a homogeneous equilibrium.
Therefore, Option (D) is false and (A), (B), and (C) are the appropriate responses.
Frequently Asked Questions-FAQs
Q1. What are the three factors that can disturb chemical equilibrium?
Answer: An equilibrium system's composition can be altered by three types of stressors: adding or withdrawing reactants or products, altering the system's overall pressure or volume, and altering its temperature.
Q2. Can you explain the treadmill-jogging girl's equilibrium state?
Answer: In this instance, the female is running in the other direction from the treadmill while maintaining the same speed. The girl's position stays the same over time as a result.
Q3. The allotropic transformation of graphite(s) into diamond(s) exists in heterogeneous equilibrium. How?
Answer: Despite having both reactant and product in the same phase, the reaction exists in heterogeneous equilibrium because solids are always immiscible in nature.
Q4. What is the basic requirement for chemical equilibrium in a system?
Answer: Chemical equilibrium is a state in which no net change in the amounts of reactants and products occurs during a reversible chemical reaction.


