-
Call Now
1800-102-2727
Calomel Electrode: Definition, Types of Electrodes, Structure and Function of Calomel Electrode, Practice Problems and FAQs:
Have you ever ordered anything online (Like gadgets, clothes, food or anything)?
I am pretty sure that you must have ordered.

Is it possible to order something online without a delivery person?
Of curse not, whatever the product you order has to be shipped to your address through a delivery person.
Similarly in electrolysis, the movement of the electrons is facilitated by the electrodes. Electrodes provide enough surface for the free receipt and delivery of necessary electrons in and out of electrolyte solution.
The electrodes are of different types depending upon the reactant and products of electrolysis. Hence, it is important to know the different types of electrodes and their basic characteristics.
Table of Content
- Definition of Electrodes
- Types of Electrodes
- Calomel Electrode
- Practice Problem
- Frequently Asked Questions (FAQs)
Definition of Electrodes
An electrode is a type of electrical conductor that makes contact with nonmetallic circuit components such as an electrolyte and, gas. Electrodes can be referred to as either an anode or a cathode depending on their ability to pass on or receive electrons in an electrochemical cell. The anode is an electrode where electrons leave the cell into the circuit and oxidation occurs. Electrons enter the cell through the cathode, where they undergo a reduction process.
An electrode of metal is not permanently fixed as an anode or cathode because it can act as an anode or a cathode depending on its electrode potential in relation to the other electrode.
Types of Electrodes
On the basis of the constitution of an electrode, electrodes are classified into the following types:
1. Metal–metal soluble salt electrode
2. Gas–ion electrode
3. Redox electrode
4. Metal–metal insoluble salt electrode
5. Calomel Electrode
6. Amalgam electrode
Calomel Electrode
The name of this electrode is derived from the mercury chloride ( calomel) used in the electrode. Calomel electrode is one of the reference electrodes used to find electrode potential of other electrodes and also in pH and potential measurements etc. Calomel electrode consists of mercury as the electrode and mercurous chloride as the electrolyte.
The electrode has nonpolarizable mercury and non- electrolyte and hence is very stable.
Structure of Calomel Electrode
The structure of the calomel electrode is made up of two glass tubes with a porous frit at the bottom of the outer tube.
The outer tube has a saturated potassium chloride solution in it. It makes contact with the solution outside in which the electrode is dipped for measurement through the frit which acts as the salt bridge. The saturated aqueous solution of KCl acts as the electrolyte.
The inner tube's bottom is lined with glass wool to allow both tubes' contents to make electrical contact with one another. The inner tube contains a paste of Hg, Hg2Cl2 and KCl.
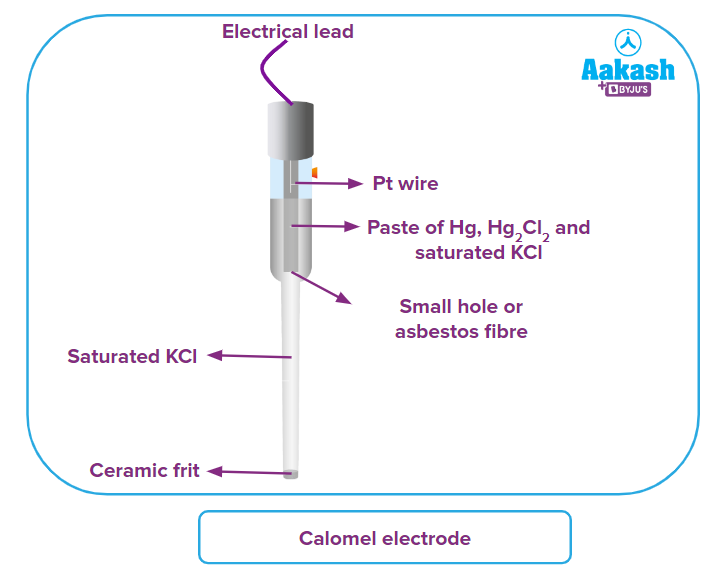
Mercury paste is distributed throughout the interior tube in a saturated potassium chloride solution.
……………(i)
…………………(ii)
Combining equation (i) & (ii) we get,
Nernst equation for this half cell
Cell representation of calomel electrode:
- Calomel electrode is a non-polarizable electrode. The saturated calomel electrode potential of 0.2444v is fairly stable. As only small currents are needed for measurement, the potential remains constant because of the relative excess amount of electrode materials. Calomel electrode can be used with more accuracy and hence highly suitable as a reference electrode over the difficult set-up of standard hydrogen electrode.
Practice Problems
Q1. Which of the following is not a reference electrode?
A. Gas Electrode
B. SHE (Standard hydrogen electrode)
C. silver-silver chloride electrode
D. Graphite electrode
Answer: (D)
Solution: An electrode with a constant and recognised electrode potential is referred to as a reference electrode. Typically, a redox system where the concentrations are constant for each redox reaction participant is used to achieve the high stability of the electrode potential. Standard Hydrogen Electrode (SHE), calomel electrode, silver-silver chloride electrode, and glass electrode are a few types of reference electrodes.
A graphite electrode is an inert electrode. Inert electrodes are those that are not involved in the reactions. There is only one function, and that is to provide an interface for reaction. You've probably heard of a galvanic cell. Both zinc and copper electrodes participate in the reactions in this cell, and electrons are taken or given to these electrodes.
Q2. The reduction potential of hydrogen half cell will be negative, if:
Answer: (C)
Solution: The reduction reaction is:
or
According to the convention, a half-cell called standard hydrogen electrode represented by , is assigned a zero potential at standard conditions.
Now
So, (PH2) > [H+]2
Q3. Given: , The potential for the cell at 298 K is:
A. 0.339 V
B. -0.339 V
C. -0.26 V
D. 0.26 V
Answer: (D)
Solution: Given:
Anode reaction:
+
Cathode reaction:
Total cell reaction:
Given and
Clearly n = 6 for this cell reaction.
According to Nernst equation:
Hence,
Q4. For the given cell, when the concentration of Zn2+ is 10 times the concentration of Cu2+, the expression of ΔG (in J mol-) is? [F is Faraday constant; R is
the gas constant; T is the temperature; E0cell = 1.1 V]
A. 2.303RT – 2.2F
B. – 2.2F
C. 2.303RT + 1.1F
D. 1.1F
Answer: (A)
Solution:
ΔG (in J mol-1) = ?
We know that
According to Nernst equation
Multiplying both sides by -nF we get
Hence,
Frequently Asked Questions (FAQs)
Q1. What is the most widely used reference electrode?
Answer: The most common type of reference electrode is a silver/silver chloride reference electrode. Because of its low half-cell potential of about +222 mV (SHE), low impedance, and lower toxicity than the calomel electrode containing mercury, silver/silver chloride is a popular choice of biological electrodes.
Q2. Can we consider a platinum electrode as a reference electrode?
Answer: No, Platinum electrodes are used as inert electrodes. Another example of an inert electrode is a graphite electrode. But Pt can be used as a reference electrode in situations where traditional reference electrodes are ineffective.
Q3. What do you understand by pH electrode?
Answer: pH electrodes are analytical sensors used to measure hydrogen potential (pH), which is the negative logarithm of hydrogen ion activity in solution. The pH of a substance is related to the concentrations of hydrogen ions [H+] and hydroxyl ions [OH-].
Q4. What are the advantages of the calomel electrode over the standard hydrogen electrode?
Answer: The ease of operation of the calomel electrode is the most advantageous. Handling gases is not only cumbersome but also is not mobile. Calomel electrode is a handy item to be used anywhere. Moreover, the potential can be directly measured without any other additional circuitry requirements.


